Remdesivir in Treatment of COVID-19: A Systematic Benefit-Risk Assessment
- PMID: 32468196
- PMCID: PMC7255634
- DOI: 10.1007/s40264-020-00952-1
Remdesivir in Treatment of COVID-19: A Systematic Benefit-Risk Assessment
Abstract
Introduction: There is a need to identify effective, safe treatments for COVID-19 (coronavirus disease) rapidly, given the current, ongoing pandemic. A systematic benefit-risk assessment was designed and conducted to examine the benefit-risk profile of remdesivir in COVID-19 patients compared with standard of care, placebo or other treatments. A key objective of this study was to provide a platform for a dynamic systematic benefit-risk evaluation, which starts with inevitably limited information (to meet the urgent unmet public health need worldwide), then update the benefit-risk evaluation as more data become available.
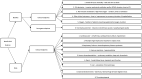
Methods: The Benefit-Risk Action Team (BRAT) framework was used to assess the overall benefit-risk of the use of remdesivir as a treatment for COVID-19 compared with standard of care, placebo or other treatments. We searched PubMed, Google Scholar and government agency websites to identify literature reporting clinical outcomes in patients taking remdesivir for COVID-19. A value tree was constructed and key benefits and risks were ranked by two clinicians in order of considered importance.
Results: Using the BRAT method, several key benefits and risks for use of remdesivir in COVID-19 compared with placebo have been identified. In one trial, the benefit of time to clinical improvement was not statistically significant (21 vs 23 days, HR 1.23, 95% CI 0.87-1.75), although the study was underpowered. In another trial, a shorter time to recovery in patients treated with remdesivir was observed (11 vs 15 days), with non-significant reduced mortality risk (8% vs 12%). Risk data were only available from one trial. This trial reported fewer serious adverse events in patients taking remdesivir (18%) compared with the placebo group (26%); however, more patients in the remdesivir group discontinued treatment as a result of an adverse event compared with those patients receiving placebo (12% vs 5%).
Conclusions: Preliminary clinical trial results suggest that there may be a favourable benefit-risk profile for remdesivir compared with placebo in severe COVID-19 infection and further data on benefits would strengthen this evaluation. There is limited safety data for remdesivir, which should be obtained in further studies. The current framework summarises the key anticipated benefits and risks for which further data are needed. Ongoing clinical trial data can be incorporated into the framework when available to provide an updated benefit-risk assessment.
Conflict of interest statement
The Drug Safety Research Unit is an independent charity (No. 327206) that works in association with the University of Portsmouth. It receives unconditional donations from pharmaceutical companies. The companies have no control over the conduct or the publication of the studies conducted by the DSRU. Gilead is providing support for an unrelated methodological project led by the DSRU as part of a large group of pharmaceutical companies, unrelated to remdesivir or any other Gilead product. They were not aware of our decision to undertake this project and provided no financial support or input on the manuscript methods and content. Miranda Davies, Vicki Osborne, Samantha Lane, Debabrata Roy, Sandeep Dhanda, Alison Evans and Saad Shakir have no other conflicts of interest to declare.
Figures
References
-
- WHO. Naming the coronavirus disease (COVID-19) and the virus that causes it; 2020.
-
- WHO. WHO announces COVID-19 outbreak a pandemic; 2020.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical