Tetrodotoxin-Sensitive Neuronal-Type Na+ Channels: A Novel and Druggable Target for Prevention of Atrial Fibrillation
- PMID: 32468902
- PMCID: PMC7429002
- DOI: 10.1161/JAHA.119.015119
Tetrodotoxin-Sensitive Neuronal-Type Na+ Channels: A Novel and Druggable Target for Prevention of Atrial Fibrillation
Abstract
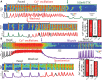
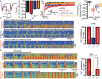
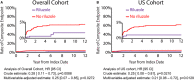
Background Atrial fibrillation (AF) is a comorbidity associated with heart failure and catecholaminergic polymorphic ventricular tachycardia. Despite the Ca2+-dependent nature of both of these pathologies, AF often responds to Na+ channel blockers. We investigated how targeting interdependent Na+/Ca2+ dysregulation might prevent focal activity and control AF. Methods and Results We studied AF in 2 models of Ca2+-dependent disorders, a murine model of catecholaminergic polymorphic ventricular tachycardia and a canine model of chronic tachypacing-induced heart failure. Imaging studies revealed close association of neuronal-type Na+ channels (nNav) with ryanodine receptors and Na+/Ca2+ exchanger. Catecholamine stimulation induced cellular and in vivo atrial arrhythmias in wild-type mice only during pharmacological augmentation of nNav activity. In contrast, catecholamine stimulation alone was sufficient to elicit atrial arrhythmias in catecholaminergic polymorphic ventricular tachycardia mice and failing canine atria. Importantly, these were abolished by acute nNav inhibition (tetrodotoxin or riluzole) implicating Na+/Ca2+ dysregulation in AF. These findings were then tested in 2 nonrandomized retrospective cohorts: an amyotrophic lateral sclerosis clinic and an academic medical center. Riluzole-treated patients adjusted for baseline characteristics evidenced significantly lower incidence of arrhythmias including new-onset AF, supporting the preclinical results. Conclusions These data suggest that nNaVs mediate Na+-Ca2+ crosstalk within nanodomains containing Ca2+ release machinery and, thereby, contribute to AF triggers. Disruption of this mechanism by nNav inhibition can effectively prevent AF arising from diverse causes.
Keywords: atrial arrhythmias; atrial fibrillation; cardiac arrhythmias; neuronal‐type Na+ channel blockade.
Figures





Similar articles
-
Tetrodotoxin-sensitive Navs contribute to early and delayed afterdepolarizations in long QT arrhythmia models.J Gen Physiol. 2018 Jul 2;150(7):991-1002. doi: 10.1085/jgp.201711909. Epub 2018 May 23. J Gen Physiol. 2018. PMID: 29793933 Free PMC article.
-
Late INa Blocker GS967 Supresses Polymorphic Ventricular Tachycardia in a Transgenic Rabbit Model of Long QT Type 2.Circ Arrhythm Electrophysiol. 2020 Aug;13(8):e006875. doi: 10.1161/CIRCEP.118.006875. Epub 2020 Jul 6. Circ Arrhythm Electrophysiol. 2020. PMID: 32628505 Free PMC article.
-
Neuronal Na+ channel blockade suppresses arrhythmogenic diastolic Ca2+ release.Cardiovasc Res. 2015 Apr 1;106(1):143-52. doi: 10.1093/cvr/cvu262. Epub 2014 Dec 23. Cardiovasc Res. 2015. PMID: 25538156 Free PMC article.
-
Targeting pathological leak of ryanodine receptors: preclinical progress and the potential impact on treatments for cardiac arrhythmias and heart failure.Expert Opin Ther Targets. 2020 Jan;24(1):25-36. doi: 10.1080/14728222.2020.1708326. Epub 2020 Jan 3. Expert Opin Ther Targets. 2020. PMID: 31869254 Free PMC article. Review.
-
Potential application of late sodium current blockade in the treatment of heart failure and atrial fibrillation.Rev Cardiovasc Med. 2009;10 Suppl 1:S46-52. Rev Cardiovasc Med. 2009. PMID: 19898288 Review.
Cited by
-
Acute GLP-1 Agonism Induces Arrhythmogenic Electrical Activity in Aged Mice Heart Through Impaired Cellular Na+ and Ca2+ Handlings: The Role of CK2 Hyperphosphorylation.Anatol J Cardiol. 2024 Dec 10;29(2):83-94. doi: 10.14744/AnatolJCardiol.2024.4719. Online ahead of print. Anatol J Cardiol. 2024. PMID: 39655941 Free PMC article.
-
Ion channel trafficking implications in heart failure.Front Cardiovasc Med. 2024 Feb 14;11:1351496. doi: 10.3389/fcvm.2024.1351496. eCollection 2024. Front Cardiovasc Med. 2024. PMID: 38420267 Free PMC article. Review.
-
Late Sodium Current of the Heart: Where Do We Stand and Where Are We Going?Pharmaceuticals (Basel). 2022 Feb 15;15(2):231. doi: 10.3390/ph15020231. Pharmaceuticals (Basel). 2022. PMID: 35215342 Free PMC article. Review.
-
Riluzole is associated with reduced risk of heart failure.Eur J Neurol. 2025 Jan;32(1):e70033. doi: 10.1111/ene.70033. Eur J Neurol. 2025. PMID: 39786321 Free PMC article.
-
NaV1.6 dysregulation within myocardial T-tubules by D96V calmodulin enhances proarrhythmic sodium and calcium mishandling.J Clin Invest. 2023 Apr 3;133(7):e152071. doi: 10.1172/JCI152071. J Clin Invest. 2023. PMID: 36821382 Free PMC article.
References
-
- Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Bailey KR, Abhayaratna WP, Seward JB, Tsang TSM. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114:119–125. - PubMed
-
- Bhuiyan ZA, van den Berg MP, van Tintelen JP, Bink‐Boelkens MT, Wiesfeld AC, Alders M, Postma AV, van Langen I, Mannens MM, Wilde AA. Expanding spectrum of human RYR2‐related disease: new electrocardiographic, structural, and genetic features. Circulation. 2007;116:1569–1576. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

