Intracranial Efficacy and Survival With Tucatinib Plus Trastuzumab and Capecitabine for Previously Treated HER2-Positive Breast Cancer With Brain Metastases in the HER2CLIMB Trial
- PMID: 32468955
- PMCID: PMC7403000
- DOI: 10.1200/JCO.20.00775
Intracranial Efficacy and Survival With Tucatinib Plus Trastuzumab and Capecitabine for Previously Treated HER2-Positive Breast Cancer With Brain Metastases in the HER2CLIMB Trial
Abstract
Purpose: In the HER2CLIMB study, patients with human epidermal growth factor receptor 2 (HER2)-positive breast cancer with brain metastases (BMs) showed statistically significant improvement in progression-free survival (PFS) with tucatinib. We describe exploratory analyses of intracranial efficacy and survival in participants with BMs.
Patients and methods: Patients were randomly assigned 2:1 to tucatinib or placebo, in combination with trastuzumab and capecitabine. All patients underwent baseline brain magnetic resonance imaging; those with BMs were classified as active or stable. Efficacy analyses were performed by applying RECIST 1.1 criteria to CNS target lesions by investigator assessment. CNS-PFS (intracranial progression or death) and overall survival (OS) were evaluated in all patients with BMs. Confirmed intracranial objective response rate (ORR-IC) was evaluated in patients with measurable intracranial disease.
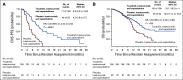
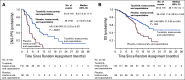
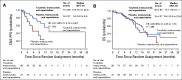
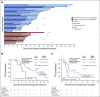
Results: There were 291 patients with BMs: 198 (48%) in the tucatinib arm and 93 (46%) in the control arm. The risk of intracranial progression or death was reduced by 68% in the tucatinib arm (hazard ratio [HR], 0.32; 95% CI, 0.22 to 0.48; P < .0001). Median CNS-PFS was 9.9 months in the tucatinib arm versus 4.2 months in the control arm. Risk of death was reduced by 42% in the tucatinib arm (OS HR, 0.58; 95% CI, 0.40 to 0.85; P = .005). Median OS was 18.1 versus 12.0 months. ORR-IC was higher in the tucatinib arm (47.3%; 95% CI, 33.7% to 61.2%) versus the control arm (20.0%; 95% CI, 5.7% to 43.7%; P = .03).
Conclusion: In patients with HER2-positive breast cancer with BMs, the addition of tucatinib to trastuzumab and capecitabine doubled ORR-IC, reduced risk of intracranial progression or death by two thirds, and reduced risk of death by nearly half. To our knowledge, this is the first regimen to demonstrate improved antitumor activity against BMs in patients with HER2-positive breast cancer in a randomized, controlled trial.
Trial registration: ClinicalTrials.gov NCT02614794.
Figures
References
-
- Bendell JC, Domchek SM, Burstein HJ, et al. Central nervous system metastases in women who receive trastuzumab-based therapy for metastatic breast carcinoma. Cancer. 2003;97:2972–2977. - PubMed
-
- Brufsky AM, Mayer M, Rugo HS, et al. Central nervous system metastases in patients with HER2-positive metastatic breast cancer: Incidence, treatment, and survival in patients from registHER. Clin Cancer Res. 2011;17:4834–4843. - PubMed
-
- Leyland-Jones B. Human epidermal growth factor receptor 2-positive breast cancer and central nervous system metastases. J Clin Oncol. 2009;27:5278–5286. - PubMed
-
- Pestalozzi BC, Holmes E, de Azambuja E, et al. CNS relapses in patients with HER2-positive early breast cancer who have and have not received adjuvant trastuzumab: A retrospective substudy of the HERA trial (BIG 1-01) Lancet Oncol. 2013;14:244–248. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

