Tepotinib in Non-Small-Cell Lung Cancer with MET Exon 14 Skipping Mutations
- PMID: 32469185
- PMCID: PMC8422679
- DOI: 10.1056/NEJMoa2004407
Tepotinib in Non-Small-Cell Lung Cancer with MET Exon 14 Skipping Mutations
Abstract
Background: A splice-site mutation that results in a loss of transcription of exon 14 in the oncogenic driver MET occurs in 3 to 4% of patients with non-small-cell lung cancer (NSCLC). We evaluated the efficacy and safety of tepotinib, a highly selective MET inhibitor, in this patient population.
Methods: In this open-label, phase 2 study, we administered tepotinib (at a dose of 500 mg) once daily in patients with advanced or metastatic NSCLC with a confirmed MET exon 14 skipping mutation. The primary end point was the objective response by independent review among patients who had undergone at least 9 months of follow-up. The response was also analyzed according to whether the presence of a MET exon 14 skipping mutation was detected on liquid biopsy or tissue biopsy.
Results: As of January 1, 2020, a total of 152 patients had received tepotinib, and 99 patients had been followed for at least 9 months. The response rate by independent review was 46% (95% confidence interval [CI], 36 to 57), with a median duration of response of 11.1 months (95% CI, 7.2 to could not be estimated) in the combined-biopsy group. The response rate was 48% (95% CI, 36 to 61) among 66 patients in the liquid-biopsy group and 50% (95% CI, 37 to 63) among 60 patients in the tissue-biopsy group; 27 patients had positive results according to both methods. The investigator-assessed response rate was 56% (95% CI, 45 to 66) and was similar regardless of the previous therapy received for advanced or metastatic disease. Adverse events of grade 3 or higher that were considered by investigators to be related to tepotinib therapy were reported in 28% of the patients, including peripheral edema in 7%. Adverse events led to permanent discontinuation of tepotinib in 11% of the patients. A molecular response, as measured in circulating free DNA, was observed in 67% of the patients with matched liquid-biopsy samples at baseline and during treatment.
Conclusions: Among patients with advanced NSCLC with a confirmed MET exon 14 skipping mutation, the use of tepotinib was associated with a partial response in approximately half the patients. Peripheral edema was the main toxic effect of grade 3 or higher. (Funded by Merck [Darmstadt, Germany]; VISION ClinicalTrials.gov number, NCT02864992.).
Copyright © 2020 Massachusetts Medical Society.
Figures

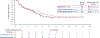

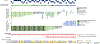
References
-
- Cortot AB, Kherrouche Z, Descarpentries C, et al. Exon 14 deleted MET receptor as a new biomarker and target in cancers. J Natl Cancer Inst 2017;109(5):djw262. - PubMed
-
- Frampton GM, Ali SM, Rosenzweig M, et al. Activation of MET via diverse exon 14 splicing alterations occurs in multiple tumor types and confers clinical sensitivity to MET inhibitors. Cancer Discov 2015;5:850–9. - PubMed
-
- Awad MM, Oxnard GR, Jackman DM, et al. MET exon 14 mutations in nonsmall-cell lung cancer are associated with advanced age and stage-dependent MET genomic amplification and c-Met overexpression. J Clin Oncol 2016;34:721–30. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
