High-Throughput Screening at the Membrane Interface Reveals Inhibitors of Amyloid-β
- PMID: 32469202
- PMCID: PMC10323872
- DOI: 10.1021/acs.biochem.0c00328
High-Throughput Screening at the Membrane Interface Reveals Inhibitors of Amyloid-β
Abstract
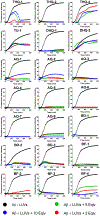
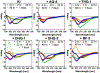
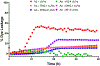
Aggregation and the formation of oligomeric intermediates of amyloid-β (Aβ) at the membrane interface of neuronal cells are implicated in the cellular toxicity and pathology of Alzheimer's disease. Small molecule compounds have been shown to suppress amyloid aggregation and cellular toxicity, but often the presence of a lipid membrane negates their activity. A high-throughput screen of 1800 small molecules was performed to search for membrane active inhibitors, and 21 primary hits were discovered. Through the use of fluorescence-based assays, transmission electron microscopy, and dot blot assays, the initial 21 primary hits were narrowed down to five lead compounds. Nuclear magnetic resonance and circular dichroism experiments were used for further confirmation of amyloid inhibition at the membrane interface and to obtain insights into the secondary structure of amyloid-β, while size exclusion chromatography was used to characterize the size of Aβ species. Lastly, dye-leakage assays allowed us to understand how the addition of the five lead compounds affected amyloid-β's ability to permeate the lipid bilayer. These results provide insights into small molecules that stabilize small amyloid species in the presence of membranes for the development of tool compounds for deeper investigations of these transient species.
Figures

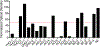

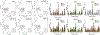
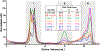
References
-
- Hamley IW The Amyloid Beta Peptide: A Chemist’s Perspective. Role in Alzheimer’s and Fibrillization. Chem. Rev 2012, 112 (10), 5147–5192. - PubMed
-
- Benilova I; Karran E; De Strooper B The Toxic Aβ Oligomer and Alzheimer’s Disease: An Emperor in Need of Clothes. Nat. Neurosci 2012, 15 (3), 349–357. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

