FOLFIRINOX for the Treatment of Advanced Gastroesophageal Cancers: A Phase 2 Nonrandomized Clinical Trial
- PMID: 32469386
- PMCID: PMC7260693
- DOI: 10.1001/jamaoncol.2020.2020
FOLFIRINOX for the Treatment of Advanced Gastroesophageal Cancers: A Phase 2 Nonrandomized Clinical Trial
Abstract
Importance: Standard first-line regimens for patients with metastatic gastroesophageal adenocarcinomas have an approximate 40% objective response rate (ORR). The combination of leucovorin, fluorouracil, irinotecan, and oxaliplatin (FOLFIRINOX) has been efficacious as first-line therapy for other gastrointestinal cancers, such as pancreatic and colon cancers.
Objective: To evaluate the clinical activity and safety of FOLFIRINOX as first-line treatment for patients with advanced gastroesophageal adenocarcinoma.
Design, setting, and participants: This is an open-label, single-arm phase 2 study of first-line FOLFIRINOX in patients with advanced gastroesophageal adenocarcinoma. Estimated sample size included 41 patients with ERBB2-negative disease with 90% power to detect an ORR of 60% or greater with α of .10. No enrollment goal was planned for ERBB2-positive patients, but they were allowed to receive trastuzumab in combination with FOLFIRINOX.
Interventions: Starting doses were fluorouracil, 400 mg/m2 bolus, followed by 2400 mg/m2 over 46 hours; leucovorin, 400 mg/m2; irinotecan, 180 mg/m2; and oxaliplatin, 85 mg/m2. Trastuzumab was administered as a 6 mg/kg loading dose, followed by 4 mg/kg every 14 days in patients with ERBB2-positive disease.
Main outcomes and measures: The primary end point was ORR by the Response Evaluation Criteria in Solid Tumors, version 1.1. Secondary end points included safety profile, progression-free survival (PFS), overall survival (OS), and duration of response.
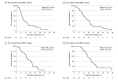
Results: From November 2013 to May 2018, 67 patients were enrolled (median [range] age, 59.0 [34-78] years; including 56 [84%] men), and 26 of 67 (39%) had ERBB2-positive disease. Median follow-up was 17.4 months. The ORR was 61%(95% CI, 44.5%-75.8%) (25 of 41) in the ERBB2-negative group and 85% (95% CI, 65.1%-95.6%) (22 of 26) in the ERBB2-positive group, including 1 patient with complete response. For ERBB2-negative patients, median PFS was 8.4 months and median OS was 15.5 months; for ERBB2-positive patients, median PFS was 13.8 months and median OS was 19.6 months. Fifty-six patients (84%) had dose modifications or treatment delays. The most common toxic effects were neutropenia (91%, n = 61), diarrhea (63%, n = 42), peripheral sensory neuropathy (61%, n = 41), and nausea (48%, n = 32), with no unexpected toxic effects.
Conclusions and relevance: The FOLFIRINOX regimen with or without trastuzumab was associated with improved ORR and PFS in patients with advanced gastroesophageal adenocarcinoma in the first-line setting. This regimen may be a reasonable therapeutic option for patients with preserved performance status.
Trial registration: ClinicalTrials.gov Identifier: NCT01928290.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

