Viral delivery of tissue nonspecific alkaline phosphatase diminishes craniosynostosis in one of two FGFR2C342Y/+ mouse models of Crouzon syndrome
- PMID: 32470062
- PMCID: PMC7259715
- DOI: 10.1371/journal.pone.0234073
Viral delivery of tissue nonspecific alkaline phosphatase diminishes craniosynostosis in one of two FGFR2C342Y/+ mouse models of Crouzon syndrome
Abstract
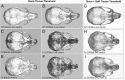
Craniosynostosis is the premature fusion of cranial bones. The goal of this study was to determine if delivery of recombinant tissue nonspecific alkaline phosphatase (TNAP) could prevent or diminish the severity of craniosynostosis in a C57BL/6 FGFR2C342Y/+ model of neonatal onset craniosynostosis or a BALB/c FGFR2C342Y/+ model of postnatal onset craniosynostosis. Mice were injected with a lentivirus encoding a mineral targeted form of TNAP immediately after birth. Cranial bone fusion as well as cranial bone volume, mineral content and density were assessed by micro CT. Craniofacial shape was measured with calipers. Alkaline phosphatase, alanine amino transferase (ALT) and aspartate amino transferase (AST) activity levels were measured in serum. Neonatal delivery of TNAP diminished craniosynostosis severity from 94% suture obliteration in vehicle treated mice to 67% suture obliteration in treated mice, p<0.02) and the incidence of malocclusion from 82.4% to 34.7% (p<0.03), with no effect on cranial bone in C57BL/6 FGFR2C342Y/+ mice. In contrast, treatment with TNAP increased cranial bone volume (p< 0.01), density (p< 0.01) and mineral content (p< 0.01) as compared to vehicle treated controls, but had no effect on craniosynostosis or malocclusion in BALB/c FGFR2C342Y/+ mice. These results indicate that postnatal recombinant TNAP enzyme therapy diminishes craniosynostosis severity in the C57BL/6 FGFR2C342Y/+ neonatal onset mouse model of Crouzon syndrome, and that effects of exogenous TNAP are genetic background dependent.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

