The thick waxy coat of mycobacteria, a protective layer against antibiotics and the host's immune system
- PMID: 32470138
- PMCID: PMC7261415
- DOI: 10.1042/BCJ20200194
The thick waxy coat of mycobacteria, a protective layer against antibiotics and the host's immune system
Abstract
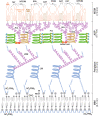
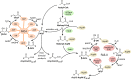
Tuberculosis, caused by the pathogenic bacterium Mycobacterium tuberculosis (Mtb), is the leading cause of death from an infectious disease, with a mortality rate of over a million people per year. This pathogen's remarkable resilience and infectivity is largely due to its unique waxy cell envelope, 40% of which comprises complex lipids. Therefore, an understanding of the structure and function of the cell wall lipids is of huge indirect clinical significance. This review provides a synopsis of the cell envelope and the major lipids contained within, including structure, biosynthesis and roles in pathogenesis.
Keywords: biosynthesis; cell wall; lipids; mycobacterium tuberculosis; mycolic acids.
© 2020 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures




References
-
- Global Tuberculosis Report (2019) World Health Organization, Geneva, Switzerland
-
- Ratledge C. (1982) Lipids: cell composition, fatty acid biosynthesis In The Biology of the Mycobacteria (Ratledge C. and Stanford J., eds), pp. 53–94, Academic Press, London, U.K
-
- Minnikin D.E. (1982) Lipids: complex lipids, their chemistry, biosynthesis and role In The Biology of Mycobacteria (Ratledge C. and Stanford J., eds), pp. 95–184, Academic Press, London, U.K
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

