The pharmacokinetics of para-aminosalicylic acid and its relationship to efficacy and intolerance
- PMID: 32470182
- PMCID: PMC7576629
- DOI: 10.1111/bcp.14395
The pharmacokinetics of para-aminosalicylic acid and its relationship to efficacy and intolerance
Abstract
Following its introduction as an antituberculosis agent close to 75 years ago, the use of para-aminosalicylic acid (PAS) has been limited by gastrointestinal intolerance and multiple formulations were produced in attempts to reduce its occurrence. More recently, an enteric-coated, granular, slow-release PAS formulation (PASER) was introduced and is now in wide-spread use for the treatment of drug-resistant tuberculosis. The current PASER dosing regimen is based on recommendations derived from older studies using a variety of different PAS formulations and relegate PAS to a role as an exclusively bacteriostatic agent. However, there is ample evidence that if sufficiently high serum concentrations are reached, PAS can be bactericidal and that intolerance following once daily dosing, that aids the achievement of such concentrations, is no worse than that following intermittent daily dosing. In particular, prevention of resistance to companion drugs appears to be dependent on the size of the single dose, and hence the peak concentrations, and not on maintaining serum levels consistently above minimum inhibitory concentration. We present a narrative review of the development of PAS formulations, dosing practices, and published data regarding pharmacokinetics and pharmacodynamics and the relationship of PAS dosage to intolerance and efficacy. Our conclusions suggests that we are at present not using PAS to its maximum ability to contribute to regimen efficacy and protect companion drugs.
Keywords: efficacy; intolerance; para-aminosalicylic acid; pharmacokinetics.
© 2020 The British Pharmacological Society.
Conflict of interest statement
All authors have completed the unified competing interest form, and declare no support from any organisation for the submitted work, and no financial interest to declare. The European and Developing Countries Clinical Trials Partnership (EDCTP) support A.A.A., grant number TRIA.2015.1102. There was no specific funding source for this manuscript.
Figures
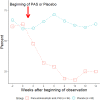
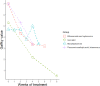
References
-
- Vallentin G, Törnell E, Beskow A, et al. PAS in pulmonary tuberculosis. Tubercle. 1950;31(1):2‐10. - PubMed
-
- Brunton LL, Knollmann BC, Hilal‐Dandan R. Goodman & Gilman's the Pharmacological Basis of Therapeutics , 13th Ed. McGraw Hill Medical; 2018.
-
- Jones JS. Intravenous P.a.S. in relation to pulmonary tuberculotherapy, with an account of 1,145 transfusions. Br J Tuberc Dis Chest. 1954;48(4):286‐297. - PubMed
-
- Dubovsky H. Correspondence with a pioneer, Jürgen Lehmann (1898‐1989), producer of the first effective antituberculosis specific. S Afr Med J. 1991;79(1):48‐50. - PubMed
-
- Lehmann J. Para‐aminosalicylic acid in the treatment of tuberculosis. Lancet. 1946;247(6384):15‐16. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

