Synthesis, Structure-Activity Relationship, and Mechanistic Studies of Aminoquinazolinones Displaying Antimycobacterial Activity
- PMID: 32470286
- PMCID: PMC7359024
- DOI: 10.1021/acsinfecdis.0c00252
Synthesis, Structure-Activity Relationship, and Mechanistic Studies of Aminoquinazolinones Displaying Antimycobacterial Activity
Abstract
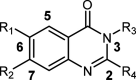
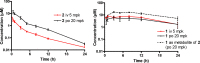
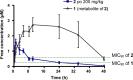
Phenotypic whole-cell screening against Mycobacterium tuberculosis (Mtb) in glycerol-alanine-salts supplemented with Tween 80 and iron (GASTE-Fe) media led to the identification of a 2-aminoquinazolinone hit compound, sulfone 1 which was optimized for solubility by replacing the sulfone moiety with a sulfoxide 2. The synthesis and structure-activity relationship (SAR) studies identified several compounds with potent antimycobacterial activity, which were metabolically stable and noncytotoxic. Compound 2 displayed favorable in vitro properties and was therefore selected for in vivo pharmacokinetic (PK) studies where it was found to be extensively metabolized to the sulfone 1. Both derivatives exhibited promising PK parameters; however, when 2 was evaluated for in vivo efficacy in an acute TB infection mouse model, it was found to be inactive. In order to understand the in vitro and in vivo discrepancy, compound 2 was subsequently retested in vitro using different Mtb strains cultured in different media. This revealed that activity was only observed in media containing glycerol and led to the hypothesis that glycerol was not used as a primary carbon source by Mtb in the mouse lungs, as has previously been observed. Support for this hypothesis was provided by spontaneous-resistant mutant generation and whole genome sequencing studies, which revealed mutations mapping to glycerol metabolizing genes indicating that the 2-aminoquinazolinones kill Mtb in vitro via a glycerol-dependent mechanism of action.
Keywords: 2-aminoquinazolinones; Mycobacterium tuberculosis; drug discovery; tuberculosis.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
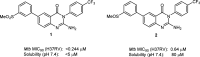
References
-
- WHO (2019) Global tuberculosis report 2019, WHO, Geneva.
-
- Liu B.; Li F.; Zhou T.; Tang X. Q.; Hu G. W. (2018) Quinoline Derivatives with Potential Activity Against Multidrug-Resistant Tuberculosis. J. Heterocycl. Chem. 55, 1863–1873. 10.1002/jhet.3241. - DOI
-
- TB Alliance (2019) FDA Approves New Treatment for Highly Drug-Resistant Forms of Tuberculosis, https://www.tballiance.org/news/fda-approves-new-treatment-highly-drug-r... (accessed 2020-02-24).
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

