Investigation and Serologic Follow-Up of Contacts of an Early Confirmed Case-Patient with COVID-19, Washington, USA
- PMID: 32470316
- PMCID: PMC7392438
- DOI: 10.3201/eid2608.201423
Investigation and Serologic Follow-Up of Contacts of an Early Confirmed Case-Patient with COVID-19, Washington, USA
Abstract
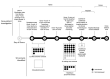
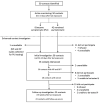
We describe the contact investigation for an early confirmed case of coronavirus disease (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), in the United States. Contacts of the case-patient were identified, actively monitored for symptoms, interviewed for a detailed exposure history, and tested for SARS-CoV-2 infection by real-time reverse transcription PCR (rRT-PCR) and ELISA. Fifty contacts were identified and 38 (76%) were interviewed, of whom 11 (29%) reported unprotected face-to-face interaction with the case-patient. Thirty-seven (74%) had respiratory specimens tested by rRT-PCR, and all tested negative. Twenty-three (46%) had ELISA performed on serum samples collected ≈6 weeks after exposure, and none had detectable antibodies to SARS-CoV-2. Among contacts who were tested, no secondary transmission was identified in this investigation, despite unprotected close interactions with the infectious case-patient.
Keywords: 2019 novel coronavirus disease; COVID-19; SARS-CoV-2; contact tracing; coronavirus; coronavirus disease; respiratory infections; serology; severe acute respiratory syndrome coronavirus 2; viruses; zoonoses.
Figures
References
-
- Centers for Disease Control and Prevention. CDC 2019-novel coronavirus (2019-nCoV) real-time RT-PCR diagnostic panel; 2020. [cited 2020 Mar 30]. https://www.fda.gov/media/134922/download
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

