AAV9-Mediated Expression of SMN Restricted to Neurons Does Not Rescue the Spinal Muscular Atrophy Phenotype in Mice
- PMID: 32470325
- PMCID: PMC7403319
- DOI: 10.1016/j.ymthe.2020.05.011
AAV9-Mediated Expression of SMN Restricted to Neurons Does Not Rescue the Spinal Muscular Atrophy Phenotype in Mice
Abstract
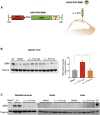
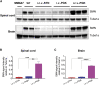
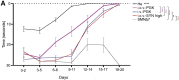
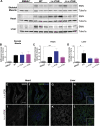
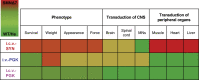
Spinal muscular atrophy (SMA) is a neuromuscular disease mainly caused by mutations or deletions in the survival of motor neuron 1 (SMN1) gene and characterized by the degeneration of motor neurons and progressive muscle weakness. A viable therapeutic approach for SMA patients is a gene replacement strategy that restores functional SMN expression using adeno-associated virus serotype 9 (AAV9) vectors. Currently, systemic or intra-cerebrospinal fluid (CSF) delivery of AAV9-SMN is being explored in clinical trials. In this study, we show that the postnatal delivery of an AAV9 that expresses SMN under the control of the neuron-specific promoter synapsin selectively targets neurons without inducing re-expression in the peripheral organs of SMA mice. However, this approach is less efficient in restoring the survival and neuromuscular functions of SMA mice than the systemic or intra-CSF delivery of an AAV9 in which SMN is placed under the control of a ubiquitous promoter. This study suggests that further efforts are needed to understand the extent to which SMN is required in neurons and peripheral organs for a successful therapeutic effect.
Keywords: AAV; SMA; SMN; gene therapy; neurons; peripheral organs; spinal muscular atrophy; synapsin promoter; ubiquitous promoter.
Copyright © 2020 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures




References
-
- Prior T.W. Perspectives and diagnostic considerations in spinal muscular atrophy. Genet. Med. 2010;12:145–152. - PubMed
-
- Prior T.W., Snyder P.J., Rink B.D., Pearl D.K., Pyatt R.E., Mihal D.C., Conlan T., Schmalz B., Montgomery L., Ziegler K. Newborn and carrier screening for spinal muscular atrophy. Am. J. Med. Genet. A. 2010;152A:1608–1616. - PubMed
-
- Crawford T.O., Pardo C.A. The neurobiology of childhood spinal muscular atrophy. Neurobiol. Dis. 1996;3:97–110. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

