β-Arrestin-Biased Allosteric Modulator of NTSR1 Selectively Attenuates Addictive Behaviors
- PMID: 32470395
- PMCID: PMC7466280
- DOI: 10.1016/j.cell.2020.04.053
β-Arrestin-Biased Allosteric Modulator of NTSR1 Selectively Attenuates Addictive Behaviors
Abstract
Small molecule neurotensin receptor 1 (NTSR1) agonists have been pursued for more than 40 years as potential therapeutics for psychiatric disorders, including drug addiction. Clinical development of NTSR1 agonists has, however, been precluded by their severe side effects. NTSR1, a G protein-coupled receptor (GPCR), signals through the canonical activation of G proteins and engages β-arrestins to mediate distinct cellular signaling events. Here, we characterize the allosteric NTSR1 modulator SBI-553. This small molecule not only acts as a β-arrestin-biased agonist but also extends profound β-arrestin bias to the endogenous ligand by selectively antagonizing G protein signaling. SBI-553 shows efficacy in animal models of psychostimulant abuse, including cocaine self-administration, without the side effects characteristic of balanced NTSR1 agonism. These findings indicate that NTSR1 G protein and β-arrestin activation produce discrete and separable physiological effects, thus providing a strategy to develop safer GPCR-targeting therapeutics with more directed pharmacological action.
Keywords: G protein-coupled recpetor; GPCR; NTSR1; PET; addiction; allosteric modulator; cocaine; dopamine; methamphetamine; neurotensin receptor 1; positron emission tomography; self-administration; β-arrestin.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests US Patents 9,868,707 and 10,118,902 relating to the chemistry of ML314, SBI-553, and their derivatives have been issued to the Sanford Burnham Prebys Medical Research Institute and Duke University (A.B.P., M.P.H., P.M., S.P., P.M., L.S.B., and M.G.C.).
Figures


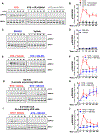



Comment in
-
Biasing Neurotensin Receptor Signaling to Arrest Psychostimulant Abuse.Cell. 2020 Jun 11;181(6):1205-1206. doi: 10.1016/j.cell.2020.05.009. Epub 2020 May 28. Cell. 2020. PMID: 32470394
References
-
- Baicy K, and London ED (2007). Corticolimbic dysregulation and chronic methamphetamine abuse. Addiction 102 Suppl 1, 5–15. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 DA029925/DA/NIDA NIH HHS/United States
- K08 HL125905/HL/NHLBI NIH HHS/United States
- R01 MH073853/MH/NIMH NIH HHS/United States
- P30 CA016086/CA/NCI NIH HHS/United States
- K01 AG041211/AG/NIA NIH HHS/United States
- R33 DA038019/DA/NIDA NIH HHS/United States
- R01 AG066184/AG/NIA NIH HHS/United States
- R00 DA048970/DA/NIDA NIH HHS/United States
- U24 CA220245/CA/NCI NIH HHS/United States
- K99 DA048970/DA/NIDA NIH HHS/United States
- R21 DA038019/DA/NIDA NIH HHS/United States
- R37 MH073853/MH/NIMH NIH HHS/United States
- F32 DA043931/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

