Ruxolitinib in treatment of severe coronavirus disease 2019 (COVID-19): A multicenter, single-blind, randomized controlled trial
- PMID: 32470486
- PMCID: PMC7250105
- DOI: 10.1016/j.jaci.2020.05.019
Ruxolitinib in treatment of severe coronavirus disease 2019 (COVID-19): A multicenter, single-blind, randomized controlled trial
Abstract
Background: Accumulating evidence proposed Janus-associated kinase (JAK) inhibitors as therapeutic targets warranting rapid investigation.
Objective: This study evaluated the efficacy and safety of ruxolitinib, a JAK1/2 inhibitor, for coronavirus disease 2019.
Methods: We conducted a prospective, multicenter, single-blind, randomized controlled phase II trial involving patients with severe coronavirus disease 2019.
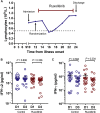
Results: Forty-three patients were randomly assigned (1:1) to receive ruxolitinib plus standard-of-care treatment (22 patients) or placebo based on standard-of-care treatment (21 patients). After exclusion of 2 patients (1 ineligible, 1 consent withdrawn) from the ruxolitinib group, 20 patients in the intervention group and 21 patients in the control group were included in the study. Treatment with ruxolitinib plus standard-of-care was not associated with significantly accelerated clinical improvement in severe patients with coronavirus disease 2019, although ruxolitinib recipients had a numerically faster clinical improvement. Eighteen (90%) patients from the ruxolitinib group showed computed tomography improvement at day 14 compared with 13 (61.9%) patients from the control group (P = .0495). Three patients in the control group died of respiratory failure, with 14.3% overall mortality at day 28; no patients died in the ruxolitinib group. Ruxolitinib was well tolerated with low toxicities and no new safety signals. Levels of 7 cytokines were significantly decreased in the ruxolitinib group in comparison to the control group.
Conclusions: Although no statistical difference was observed, ruxolitinib recipients had a numerically faster clinical improvement. Significant chest computed tomography improvement, a faster recovery from lymphopenia, and favorable side-effect profile in the ruxolitinib group were encouraging and informative to future trials to test efficacy of ruxolitinib in a larger population.
Keywords: COVID-19; Ruxolitinib; cytokine storm; efficacy; randomized controlled trial; safety.
Copyright © 2020 American Academy of Allergy, Asthma & Immunology. Published by Elsevier Inc. All rights reserved.
Figures



Comment in
-
Whether the timing of patient randomization interferes with the assessment of the efficacy of ruxolitinib for severe COVID-19.J Allergy Clin Immunol. 2020 Dec;146(6):1453. doi: 10.1016/j.jaci.2020.09.002. Epub 2020 Sep 17. J Allergy Clin Immunol. 2020. PMID: 32980157 Free PMC article. No abstract available.
-
Reply.J Allergy Clin Immunol. 2020 Dec;146(6):1453-1454. doi: 10.1016/j.jaci.2020.07.037. Epub 2020 Sep 18. J Allergy Clin Immunol. 2020. PMID: 32980158 Free PMC article. No abstract available.
References
-
- Mahase E. Covid-19: WHO declares pandemic because of “alarming levels” of spread, severity, and inaction. BMJ. 2020;368:m1036. - PubMed
-
- Lai CC, Liu YH, Wang CY, Wang YH, Hsueh SC, Yen MY, et al. Asymptomatic carrier state, acute respiratory disease, and pneumonia due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): facts and myths [published online ahead of print March 4, 2020]. J Microbiol Immunol Infect. . - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

