The role of Th17 immunity in chronic ocular surface disorders
- PMID: 32470612
- PMCID: PMC8552272
- DOI: 10.1016/j.jtos.2020.05.009
The role of Th17 immunity in chronic ocular surface disorders
Abstract
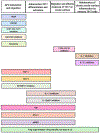
Th17 cells have been implicated in the pathogenesis of numerous inflammatory and autoimmune conditions. At the ocular surface, Th17 cells have been identified as key effector cells in chronic ocular surface disease. Evidence from murine studies indicates that following differentiation and expansion, Th17 cells migrate from the lymphoid tissues to the eye, where they release inflammatory cytokines including, but not limited to, their hallmark cytokine IL-17A. As the acute phase subsides, a population of long-lived memory Th17 cells persist, which predispose hosts both to chronic inflammation and severe exacerbations of disease; of great interest is the small subset of Th17/1 cells that secrete both IL-17A and IFN-γ in acute-on-chronic disease exacerbation. Over the past decade, substantial progress has been made in deciphering how Th17 cells interact with the immune and neuroimmune pathways that mediate chronic ocular surface disease. Here, we review (i) the evidence for Th17 immunity in chronic ocular surface disease, (ii) regulatory mechanisms that constrain the Th17 immune response, and (iii) novel therapeutic strategies targeting Th17 cells.
Keywords: Chronic ocular surface disorders; Dry eye disease; IL-17; Immunological memory; Immunoregulation; Th17.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

