Retinal Ganglion Cell Survival and Axon Regeneration after Optic Nerve Transection is Driven by Cellular Intravitreal Sciatic Nerve Grafts
- PMID: 32471105
- PMCID: PMC7349876
- DOI: 10.3390/cells9061335
Retinal Ganglion Cell Survival and Axon Regeneration after Optic Nerve Transection is Driven by Cellular Intravitreal Sciatic Nerve Grafts
Abstract
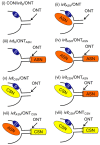
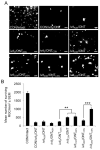
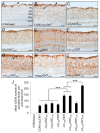
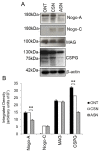
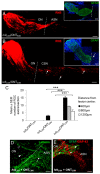
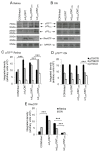
Neurotrophic factors (NTF) secreted by Schwann cells in a sciatic nerve (SN) graft promote retinal ganglion cell (RGC) axon regeneration after either transplantation into the vitreous body of the eye or anastomosis to the distal stump of a transected optic nerve. In this study, we investigated the neuroprotective and growth stimulatory properties of SN grafts in which Schwann cells had been killed (acellular SN grafts, ASN) or remained intact (cellular SN grafts, CSN). We report that both intravitreal (ivit) implanted and optic nerve anastomosed CSN promote RGC survival and when simultaneously placed in both sites, they exert additive RGC neuroprotection. CSN and ASN were rich in myelin-associated glycoprotein (MAG) and axon growth-inhibitory ligand common to both the central nervous system (CNS) and peripheral nervous system (PNS) myelin. The penetration of the few RGC axons regenerating into an ASN at an optic nerve transection (ONT) site is limited into the proximal perilesion area, but is increased >2-fold after ivit CSN implantation and increased 5-fold into a CSN optic nerve graft after ivit CSN implantation, potentiated by growth disinhibition through the regulated intramembranous proteolysis (RIP) of p75NTR (the signalling trans-membrane moiety of the nogo-66 trimeric receptor that binds MAG and associated suppression of RhoGTP). Mϋller cells/astrocytes become reactive after all treatments and maximally after simultaneous ivit and optic nerve CSN/ASN grafting. We conclude that simultaneous ivit CSN plus optic nerve CSN support promotes significant RGC survival and axon regeneration into CSN optic nerve grafts, despite being rich in axon growth inhibitory molecules. RGC axon regeneration is probably facilitated through RIP of p75NTR, which blinds axons to myelin-derived axon growth-inhibitory ligands present in optic nerve grafts.
Keywords: CNS; axon regeneration; neuroprotection; neurotrophic factors; optic nerve; optic nerve transection; peripheral nerve grafts; retinal ganglion cells.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

Similar articles
-
Schwann cell-derived factor-induced modulation of the NgR/p75NTR/EGFR axis disinhibits axon growth through CNS myelin in vivo and in vitro.Brain. 2006 Jun;129(Pt 6):1517-33. doi: 10.1093/brain/awl080. Epub 2006 Apr 13. Brain. 2006. PMID: 16613894
-
Differential effects of intravitreal optic nerve and sciatic nerve grafts on the survival of retinal ganglion cells and the regeneration of their axons.J Neurocytol. 2001 Dec;30(12):983-91. doi: 10.1023/a:1021884606771. J Neurocytol. 2001. PMID: 12626880
-
Peripheral nerve explants grafted into the vitreous body of the eye promote the regeneration of retinal ganglion cell axons severed in the optic nerve.J Neurocytol. 1996 Feb;25(2):147-70. doi: 10.1007/BF02284793. J Neurocytol. 1996. PMID: 8699196
-
Regeneration of axons in the visual system.Restor Neurol Neurosci. 2008;26(2-3):147-74. Restor Neurol Neurosci. 2008. PMID: 18820408 Review.
-
Neuroinflammation, Microglia and Implications for Retinal Ganglion Cell Survival and Axon Regeneration in Traumatic Optic Neuropathy.Front Immunol. 2022 Mar 4;13:860070. doi: 10.3389/fimmu.2022.860070. eCollection 2022. Front Immunol. 2022. PMID: 35309305 Free PMC article. Review.
Cited by
-
Longitudinal Observation of Retinal Response to Optic Nerve Transection in Rats Using Visible Light Optical Coherence Tomography.Invest Ophthalmol Vis Sci. 2023 Apr 3;64(4):17. doi: 10.1167/iovs.64.4.17. Invest Ophthalmol Vis Sci. 2023. PMID: 37057973 Free PMC article.
-
Advances in novel biomaterials combined with traditional Chinese medicine rehabilitation technology in treatment of peripheral nerve injury.Front Neurol. 2024 Jun 13;15:1421772. doi: 10.3389/fneur.2024.1421772. eCollection 2024. Front Neurol. 2024. PMID: 38938781 Free PMC article. Review.
-
Purified regenerating retinal neurons reveal regulatory role of DNA methylation-mediated Na+/K+-ATPase in murine axon regeneration.Commun Biol. 2023 Jan 30;6(1):120. doi: 10.1038/s42003-023-04463-4. Commun Biol. 2023. PMID: 36717618 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

