Increase in the Number of Bone Marrow Osteoclast Precursors at Different Skeletal Sites, Particularly in Long Bone and Jaw Marrow in Mice Lacking IL-1RA
- PMID: 32471111
- PMCID: PMC7312984
- DOI: 10.3390/ijms21113774
Increase in the Number of Bone Marrow Osteoclast Precursors at Different Skeletal Sites, Particularly in Long Bone and Jaw Marrow in Mice Lacking IL-1RA
Abstract
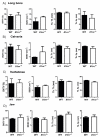
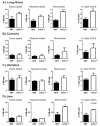
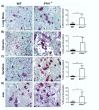
Recently, it was shown that interleukin-1β (IL-1β) has diverse stimulatory effects on different murine long bone marrow osteoclast precursors (OCPs) in vitro. In this study, interleukin-1 receptor antagonist deficient (Il1rn-/-) and wild-type (WT) mice were compared to investigate the effects of enhanced IL-1 signaling on the composition of OCPs in long bone, calvaria, vertebra, and jaw. Bone marrow cells were isolated from these sites and the percentage of early blast (CD31hi Ly-6C-), myeloid blast (CD31+ Ly-6C+), and monocyte (CD31- Ly-6Chi) OCPs was assessed by flow cytometry. At the time-point of cell isolation, Il1rn-/- mice showed no inflammation or bone destruction yet as determined by histology and microcomputed tomography. However, Il1rn-/- mice had an approximately two-fold higher percentage of OCPs in long bone and jaw marrow compared to WT. Conversely, vertebrae and calvaria marrow contained a similar composition of OCPs in both strains. Bone marrow cells were cultured with macrophage colony stimulating factor (M-CSF) and receptor of NfκB ligand (RANKL) on bone slices to assess osteoclastogenesis and on calcium phosphate-coated plates to analyze mineral dissolution. Deletion of Il1rn increased osteoclastogenesis from long bone, calvaria, and jaw marrows, and all Il1rn-/- cultures showed increased mineral dissolution compared to WT. However, osteoclast markers increased exclusively in Il1rn-/- osteoclasts from long bone and jaw. Collectively, these findings indicate that a lack of IL-1RA increases the numbers of OCPs in vivo, particularly in long bone and jaw, where rheumatoid arthritis and periodontitis develop. Thus, increased bone loss at these sites may be triggered by a larger pool of OCPs due to the disruption of IL-1 inhibitors.
Keywords: IL-1 signaling; calvaria; early blast; jaw; long bone; monocyte; myeoloid blast; osteoclast; osteoclast heterogeneity; osteoclast precursor; vertebrae.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
IL-1β differently stimulates proliferation and multinucleation of distinct mouse bone marrow osteoclast precursor subsets.J Leukoc Biol. 2016 Sep;100(3):513-23. doi: 10.1189/jlb.1A1215-543R. Epub 2016 Mar 8. J Leukoc Biol. 2016. PMID: 26957213
-
Jaw and long bone marrows have a different osteoclastogenic potential.Calcif Tissue Int. 2011 Jan;88(1):63-74. doi: 10.1007/s00223-010-9418-4. Epub 2010 Sep 23. Calcif Tissue Int. 2011. PMID: 20862464 Free PMC article.
-
TNF-α has both stimulatory and inhibitory effects on mouse monocyte-derived osteoclastogenesis.J Cell Physiol. 2017 Dec;232(12):3273-3285. doi: 10.1002/jcp.26024. Epub 2017 Jul 17. J Cell Physiol. 2017. PMID: 28543070 Free PMC article.
-
Presence of osteoclast precursors in colonies cloned in the presence of hematopoietic colony-stimulating factors.Exp Hematol. 2001 Jan;29(1):68-76. doi: 10.1016/s0301-472x(00)00626-3. Exp Hematol. 2001. PMID: 11164107
-
Regulation of osteoclast precursors in inflammatory bone loss.Curr Opin Investig Drugs. 2009 Nov;10(11):1195-203. Curr Opin Investig Drugs. 2009. PMID: 19876787 Review.
Cited by
-
IL-1 Receptor Antagonist Anakinra Inhibits the Effect of IL-1β- Mediated Osteoclast Formation by Periodontal Ligament Fibroblasts.Biology (Basel). 2025 Feb 28;14(3):250. doi: 10.3390/biology14030250. Biology (Basel). 2025. PMID: 40136507 Free PMC article.
-
Effects of Puerarin Combined with PLGA/TCP/Puerarin on Osteocalcin and Sialoprotein of Mandibular Defects.Contrast Media Mol Imaging. 2022 Sep 6;2022:5177419. doi: 10.1155/2022/5177419. eCollection 2022. Contrast Media Mol Imaging. 2022. PMID: 36128172 Free PMC article.
-
The emerging role of osteoclasts in the treatment of bone metastases: rationale and recent clinical evidence.Front Oncol. 2024 Aug 1;14:1445025. doi: 10.3389/fonc.2024.1445025. eCollection 2024. Front Oncol. 2024. PMID: 39148909 Free PMC article. Review.
-
Retrospective Case-Control Study Genes Related to Bone Metabolism That Justify the Condition of Periodontal Disease and Failure of Dental Implants in Patients with down Syndrome.Int J Mol Sci. 2023 Apr 23;24(9):7723. doi: 10.3390/ijms24097723. Int J Mol Sci. 2023. PMID: 37175429 Free PMC article.
-
Inhibitory Effects of Astaxanthin on CML-HSA-Induced Inflammatory and RANKL-Induced Osteoclastogenic Gene Expression in RAW 264.7 Cells.Biomedicines. 2021 Dec 27;10(1):54. doi: 10.3390/biomedicines10010054. Biomedicines. 2021. PMID: 35052734 Free PMC article.
References
-
- De Vries T.J., El Bakkali I., Kamradt T., Schett G., Jansen I.D.C., D′Amelio P. What Are the Peripheral Blood Determinants for Increased Osteoclast Formation in the Various Inflammatory Diseases Associated With Bone Loss? Front. Immunol. 2019;10:505. doi: 10.3389/fimmu.2019.00505. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

