Intranasal Niosomal In Situ Gel as a Promising Approach for Enhancing Flibanserin Bioavailability and Brain Delivery: In Vitro Optimization and Ex Vivo/ In Vivo Evaluation
- PMID: 32471119
- PMCID: PMC7356232
- DOI: 10.3390/pharmaceutics12060485
Intranasal Niosomal In Situ Gel as a Promising Approach for Enhancing Flibanserin Bioavailability and Brain Delivery: In Vitro Optimization and Ex Vivo/ In Vivo Evaluation
Retraction in
-
RETRACTED: Fahmy et al. Intranasal Niosomal In Situ Gel as a Promising Approach for Enhancing Flibanserin Bioavailability and Brain Delivery: In Vitro Optimization and Ex Vivo/In Vivo Evaluation. Pharmaceutics 2020, 12, 485.Pharmaceutics. 2024 Jan 29;16(2):189. doi: 10.3390/pharmaceutics16020189. Pharmaceutics. 2024. PMID: 38399352 Free PMC article.
Abstract
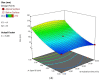
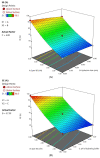
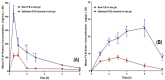
Flibanserin (FLB) is a multifunctional serotonergic agent that was recently approved by the FDA for the oral treatment of premenopausal women with hypoactive sexual desire disorder. FLB is a centrally acting drug that has a low oral bioavailability of 33% owing to its exposure to the hepatic first-pass effect, as well as its pH-dependent solubility, which could be an obstacle hindering the drug dissolution and absorption via mucosal barriers. Thus, this work aimed at overcoming the aforementioned drawbacks and promoting the nose-to-brain delivery of FLB via the formulation of an intra-nasal in situ niosomal gel. The Box-Behnken design was employed to study the impact of Span® 85 concentration (X1), hydration time (X2), and pH of the hydrating buffer (X3) on the vesicle size and drug entrapment. The optimized formulation exhibited a spherical shape with a vesicular size of 46.35 nm and entrapment efficiency of 92.48%. The optimized FLB niosomes integrated into gellan gum-based in situ gel exhibited enhanced ex vivo permeation and improved plasma and brain concentrations after nasal administration in rats compared to raw FLB. These findings highlight the capability of the proposed intra-nasal FLB niosomal in situ gel to boost the drug bioavailability and to promote its direct delivery to the brain.
Keywords: Box-Behnken; Span® 85; cholesterol; ex vivo permeation; flibanserin; gellan gum; niosomes; pharmacokinetics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









Similar articles
-
Optimized Nanostructured Lipid Carriers Integrated into In Situ Nasal Gel for Enhancing Brain Delivery of Flibanserin.Int J Nanomedicine. 2020 Jul 24;15:5253-5264. doi: 10.2147/IJN.S258791. eCollection 2020. Int J Nanomedicine. 2020. Retraction in: Int J Nanomedicine. 2023 Apr 28;18:2253-2254. doi: 10.2147/IJN.S419019. PMID: 32801690 Free PMC article. Retracted.
-
Application of Nanopharmaceutics for Flibanserin Brain Delivery Augmentation Via the Nasal Route.Nanomaterials (Basel). 2020 Jun 29;10(7):1270. doi: 10.3390/nano10071270. Nanomaterials (Basel). 2020. Retraction in: Nanomaterials (Basel). 2024 Jan 12;14(2):174. doi: 10.3390/nano14020174. PMID: 32610539 Free PMC article. Retracted.
-
Formulation and Evaluation of Niosomal in situ Nasal Gel of a Serotonin Receptor Agonist, Buspirone Hydrochloride for the Brain Delivery via Intranasal Route.Pharm Nanotechnol. 2018;6(1):69-78. doi: 10.2174/2211738506666180130105919. Pharm Nanotechnol. 2018. PMID: 29380709
-
Qbd-Based Approach to Optimize Niosomal Gel of Levosulpiride for Transdermal Drug Delivery.Gels. 2023 Mar 10;9(3):213. doi: 10.3390/gels9030213. Gels. 2023. PMID: 36975662 Free PMC article.
-
Flibanserin.2018 Jan 26. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012–. 2018 Jan 26. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012–. PMID: 31643404 Free Books & Documents. Review.
Cited by
-
Fluvastatin-Loaded Emulsomes Exhibit Improved Cytotoxic and Apoptosis in Prostate Cancer Cells.AAPS PharmSciTech. 2021 Jun 14;22(5):177. doi: 10.1208/s12249-021-02021-x. AAPS PharmSciTech. 2021. Retraction in: AAPS PharmSciTech. 2023 Jun 2;24(5):128. doi: 10.1208/s12249-023-02591-y. PMID: 34128106 Retracted.
-
Andrographolide nanophytosomes exhibit enhanced cellular delivery and pro-apoptotic activities in HepG2 liver cancer cells.Drug Deliv. 2023 Dec;30(1):2174209. doi: 10.1080/10717544.2023.2174209. Drug Deliv. 2023. PMID: 36762548 Free PMC article.
-
Spanlastics as a Potential Platform for Enhancing the Brain Delivery of Flibanserin: In Vitro Response-Surface Optimization and In Vivo Pharmacokinetics Assessment.Pharmaceutics. 2022 Nov 28;14(12):2627. doi: 10.3390/pharmaceutics14122627. Pharmaceutics. 2022. PMID: 36559120 Free PMC article.
-
Merging Experimental Design and Nanotechnology for the Development of Optimized Simvastatin Spanlastics: A Promising Combined Strategy for Augmenting the Suppression of Various Human Cancer Cells.Pharmaceutics. 2022 May 9;14(5):1024. doi: 10.3390/pharmaceutics14051024. Pharmaceutics. 2022. PMID: 35631609 Free PMC article.
-
Green Nanoemulsion Stabilized by In Situ Self-Assembled Natural Oil/Native Cyclodextrin Complexes: An Eco-Friendly Approach for Enhancing Anticancer Activity of Costunolide against Lung Cancer Cells.Pharmaceutics. 2022 Jan 19;14(2):227. doi: 10.3390/pharmaceutics14020227. Pharmaceutics. 2022. PMID: 35213960 Free PMC article.
References
-
- Allers K.A., Dremencov E., Ceci A., Flik G., Ferger B., Cremers T.I.F.H., Ittrich C., Sommer B. Acute and repeated flibanserin administration in female rats modulates monoamines differentially across brain areas: A microdialysis study. J. Sex. Med. 2010;7:1757–1767. doi: 10.1111/j.1743-6109.2010.01763.x. - DOI - PubMed
-
- El-Kattan A., Varma M. Oral Absorption, Intestinal Metabolism and Human Oral Bioavailability. In: James Paxton, editor. Topics on Drug Metabolism. IntechOpen; London, UK: 2012.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

