Vav1 is Essential for HIF-1α Activation via a Lysosomal VEGFR1-Mediated Degradation Mechanism in Endothelial Cells
- PMID: 32471123
- PMCID: PMC7352305
- DOI: 10.3390/cancers12061374
Vav1 is Essential for HIF-1α Activation via a Lysosomal VEGFR1-Mediated Degradation Mechanism in Endothelial Cells
Abstract
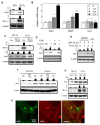
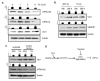
The vascular response to hypoxia and ischemia is essential for maintaining homeostasis during stressful conditions and is particularly critical for vital organs such as the heart. Hypoxia-inducible factor-1 (HIF-1) is a central regulator of the response to hypoxia by activating transcription of numerous target genes, including vascular endothelial growth factor (VEGF). Here we identify the guanine nucleotide exchange factor (GEF) Vav1, a regulator of the small Rho-GTPase and cell signaling in endothelial cells, as a key vascular regulator of hypoxia. We show that Vav1 is present in the vascular endothelium and is essential for HIF-1 activation under hypoxia. So, we hypothesized that Vav1 could be a key regulator of HIF-1 signaling. In our findings, Vav1 regulates HIF-1α stabilization through the p38/Siah2/PHD3 pathway. In normoxia, Vav1 binds to vascular endothelial growth factor receptor 1 (VEGFR1), which directs Vav1 to lysosomes for degradation. In contrast, hypoxia upregulates Vav1 protein levels by inhibiting lysosomal degradation, which is analogous to HIF-1α regulation by hypoxia: both proteins are constitutively produced and degraded in normoxia allowing for a rapid response when stress occurs. Consequently, hypoxia rapidly stabilizes Vav1, which is required for HIF-1α accumulation. This shows that Vav1 is the key mediator controlling the stabilization of HIF1α in hypoxic conditions. With this finding, we report a novel pathway to stabilize HIF-1, which shows a possible reason why clinical trials targeting HIF-1 has not been effective. Targeting Vav1 can be the new approach to overcome hypoxic tumors.
Keywords: HIF-1α; VEGFR; Vav1; endothelial cell; hypoxia; protein degradation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
The Rho/Rac Guanine Nucleotide Exchange Factor Vav1 Regulates Hif-1α and Glut-1 Expression and Glucose Uptake in the Brain.Int J Mol Sci. 2020 Feb 17;21(4):1341. doi: 10.3390/ijms21041341. Int J Mol Sci. 2020. PMID: 32079227 Free PMC article.
-
Identification of a novel small-molecule inhibitor of the hypoxia-inducible factor 1 pathway.Cancer Res. 2005 Jan 15;65(2):605-12. Cancer Res. 2005. PMID: 15695405
-
Gastric epithelial reactive oxygen species prevent normoxic degradation of hypoxia-inducible factor-1alpha in gastric cancer cells.Clin Cancer Res. 2003 Jan;9(1):433-40. Clin Cancer Res. 2003. PMID: 12538497
-
Critical role of hypoxia sensor--HIF-1α in VEGF gene activation. Implications for angiogenesis and tissue injury healing.Curr Med Chem. 2012;19(1):90-7. doi: 10.2174/092986712803413944. Curr Med Chem. 2012. PMID: 22300081 Review.
-
Vasculogenesis and angiogenesis initiation under normoxic conditions through Wnt/β-catenin pathway in gliomas.Rev Neurosci. 2018 Jan 26;29(1):71-91. doi: 10.1515/revneuro-2017-0032. Rev Neurosci. 2018. PMID: 28822229 Review.
Cited by
-
Vav1 promotes inflammation and neuronal apoptosis in cerebral ischemia/reperfusion injury by upregulating microglial and NLRP3 inflammasome activation.Neural Regen Res. 2023 Nov;18(11):2436-2442. doi: 10.4103/1673-5374.371368. Neural Regen Res. 2023. PMID: 37282474 Free PMC article.
-
HIF1α/VEGF Feedback Loop Contributes to 5-Fluorouracil Resistance.Front Pharmacol. 2022 Mar 9;13:851401. doi: 10.3389/fphar.2022.851401. eCollection 2022. Front Pharmacol. 2022. PMID: 35355718 Free PMC article.
-
A ROS-Responsive Lipid Nanoparticles Release Multifunctional Hydrogel Based on Microenvironment Regulation Promotes Infected Diabetic Wound Healing.Adv Sci (Weinh). 2024 Nov;11(43):e2403219. doi: 10.1002/advs.202403219. Epub 2024 Sep 23. Adv Sci (Weinh). 2024. PMID: 39308241 Free PMC article.
-
Glucose-Sensing Carbohydrate Response Element-Binding Protein in the Pathogenesis of Diabetic Retinopathy.Cells. 2025 Jan 13;14(2):107. doi: 10.3390/cells14020107. Cells. 2025. PMID: 39851533 Free PMC article.
-
Hematopoietic Cells Influence Vascular Development in the Retina.Cells. 2022 Oct 13;11(20):3207. doi: 10.3390/cells11203207. Cells. 2022. PMID: 36291075 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

