Transposon-Based CAR T Cells in Acute Leukemias: Where are We Going?
- PMID: 32471151
- PMCID: PMC7349235
- DOI: 10.3390/cells9061337
Transposon-Based CAR T Cells in Acute Leukemias: Where are We Going?
Abstract
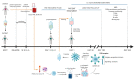
Chimeric Antigen Receptor (CAR) T-cell therapy has become a new therapeutic reality for refractory and relapsed leukemia patients and is also emerging as a potential therapeutic option in solid tumors. Viral vector-based CAR T-cells initially drove these successful efforts; however, high costs and cumbersome manufacturing processes have limited the widespread clinical implementation of CAR T-cell therapy. Here we will discuss the state of the art of the transposon-based gene transfer and its application in CAR T immunotherapy, specifically focusing on the Sleeping Beauty (SB) transposon system, as a valid cost-effective and safe option as compared to the viral vector-based systems. A general overview of SB transposon system applications will be provided, with an update of major developments, current clinical trials achievements and future perspectives exploiting SB for CAR T-cell engineering. After the first clinical successes achieved in the context of B-cell neoplasms, we are now facing a new era and it is paramount to advance gene transfer technology to fully exploit the potential of CAR T-cells towards next-generation immunotherapy.
Keywords: CAR T-cells; acute leukemia; gene transfer; immunotherapy; sleeping beauty; transposon.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Lee D.W., Kochenderfer J.N., Stetler-Stevenson M., Cui Y.K., Delbrook C., A Feldman S., Fry T.J., Orentas R., Sabatino M., Shah N.N., et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: A phase 1 dose-escalation trial. Lancet. 2015;385:517–528. doi: 10.1016/S0140-6736(14)61403-3. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

