The Pluripotency Factor Nanog Protects against Neuronal Amyloid β-Induced Toxicity and Oxidative Stress through Insulin Sensitivity Restoration
- PMID: 32471175
- PMCID: PMC7348813
- DOI: 10.3390/cells9061339
The Pluripotency Factor Nanog Protects against Neuronal Amyloid β-Induced Toxicity and Oxidative Stress through Insulin Sensitivity Restoration
Abstract
Amyloid β (Aβ) is a peptide fragment of the amyloid precursor protein that triggers the progression of Alzheimer's Disease (AD). It is believed that Aβ contributes to neurodegeneration in several ways, including mitochondria dysfunction, oxidative stress and brain insulin resistance. Therefore, protecting neurons from Aβ-induced neurotoxicity is an effective strategy for attenuating AD pathogenesis. Recently, applications of stem cell-based therapies have demonstrated the ability to reduce the progression and outcome of neurodegenerative diseases. Particularly, Nanog is recognized as a stem cell-related pluripotency factor that enhances self-renewing capacities and helps reduce the senescent phenotypes of aged neuronal cells. However, whether the upregulation of Nanog can be an effective approach to alleviate Aβ-induced neurotoxicity and senescence is not yet understood. In the present study, we transiently overexpressed Nanog-both in vitro and in vivo-and investigated the protective effects and underlying mechanisms against Aβ. We found that overexpression of Nanog is responsible for attenuating Aβ-triggered neuronal insulin resistance, which restores cell survival through reducing intracellular mitochondrial superoxide accumulation and cellular senescence. In addition, upregulation of Nanog expression appears to increase secretion of neurotrophic factors through activation of the Nrf2 antioxidant defense pathway. Furthermore, improvement of memory and learning were also observed in rat model of Aβ neurotoxicity mediated by upregulation of Nanog in the brain. Taken together, our study suggests a potential role for Nanog in attenuating the neurotoxic effects of Aβ, which in turn, suggests that strategies to enhance Nanog expression may be used as a novel intervention for reducing Aβ neurotoxicity in the AD brain.
Keywords: Nanog; amyloid β; insulin signaling; oxidative stress; senescence.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript or in the decision to publish the results.
Figures


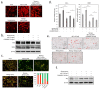
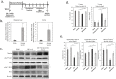
References
-
- Lin C.L., Huang W.N., Li H.H., Huang C.N., Hsieh S., Lai C., Lu F.J. Hydrogen-rich water attenuates amyloid beta-induced cytotoxicity through upregulation of Sirt1-FoxO3a by stimulation of AMP-activated protein kinase in SK-N-MC cells. Chem. -Biol. Interact. 2015;240:12–21. doi: 10.1016/j.cbi.2015.07.013. - DOI - PubMed
-
- Marchant N.L., Reed B.R., Sanossian N., Madison C.M., Kriger S., Dhada R., Mack W.J., DeCarli C., Weiner M.W., Mungas D.M., et al. The aging brain and cognition: Contribution of vascular injury and abeta to mild cognitive dysfunction. Jama Neurol. 2013;70:488–495. doi: 10.1001/2013.jamaneurol.405. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

