Alteration of Electron Acceptor Preferences in the Oxidative Half-Reaction of Flavin-Dependent Oxidases and Dehydrogenases
- PMID: 32471202
- PMCID: PMC7312611
- DOI: 10.3390/ijms21113797
Alteration of Electron Acceptor Preferences in the Oxidative Half-Reaction of Flavin-Dependent Oxidases and Dehydrogenases
Abstract
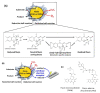
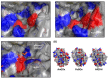
In this review, recent progress in the engineering of the oxidative half-reaction of flavin-dependent oxidases and dehydrogenases is discussed, considering their current and future applications in bioelectrochemical studies, such as for the development of biosensors and biofuel cells. There have been two approaches in the studies of oxidative half-reaction: engineering of the oxidative half-reaction with oxygen, and engineering of the preference for artificial electron acceptors. The challenges for engineering oxidative half-reactions with oxygen are further categorized into the following approaches: (1) mutation to the putative residues that compose the cavity where oxygen may be located, (2) investigation of the vicinities where the reaction with oxygen may take place, and (3) investigation of possible oxygen access routes to the isoalloxazine ring. Among these approaches, introducing a mutation at the oxygen access route to the isoalloxazine ring represents the most versatile and effective strategy. Studies to engineer the preference of artificial electron acceptors are categorized into three different approaches: (1) engineering of the charge at the residues around the substrate entrance, (2) engineering of a cavity in the vicinity of flavin, and (3) decreasing the glycosylation degree of enzymes. Among these approaches, altering the charge in the vicinity where the electron acceptor may be accessed will be most relevant.
Keywords: bioelectrochemistry; dehydrogenase; electron acceptor; enzyme engineering; flavin adenine dinucleotide; flavin mononucleotide; oxidase; oxidative half-reaction; oxygen; oxygen accessible pathway.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
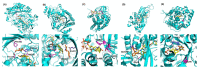


References
-
- Martens N., Hindle A., Hall E.A.H. An assessment of mediators as oxidants for glucose oxidase in the presence of oxygen. Biosens. Bioelectron. 1995;10:393–403. doi: 10.1016/0956-5663(95)96857-U. - DOI
-
- Trampitsch C., Slavica A., Riethorst W., Nidetzky B. Reaction of Trigonopsis variabilis D-amino acid oxidase with 2,6-dichloroindophenol: Kinetic characterisation and development of an oxygen-independent assay of the enzyme activity. J. Mol. Catal. B Enzym. 2005;32:271–278. doi: 10.1016/j.molcatb.2004.12.011. - DOI
-
- Kenausis G., Taylor C., Katakis I., Heller A. ‘Wiring’ of glucose oxidase and lactate oxidase within a hydrogel made with poly(vinyl pyridine) complexed with [Os(4,4[prime or minute]-dimethoxy-2,2[prime or minute]-bipyridine)2Cl]+/2+ J. Chem. Soc. Faraday Trans. 1996;92:4131–4136. doi: 10.1039/FT9969204131. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

