Characterization of Scardovia wiggsiae Biofilm by Original Scanning Electron Microscopy Protocol
- PMID: 32471210
- PMCID: PMC7355790
- DOI: 10.3390/microorganisms8060807
Characterization of Scardovia wiggsiae Biofilm by Original Scanning Electron Microscopy Protocol
Abstract
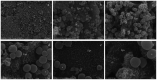
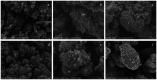
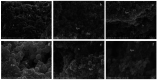
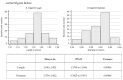
Early childhood caries (ECC) is a severe manifestation of carious pathology with rapid and disruptive progression. The ECC microbiota includes a wide variety of bacterial species, among which is an anaerobic newly named species, Scardovia wiggsiae, a previously unidentified Bifidobacterium. Our aim was to provide the first ultrastructural characterization of S. wiggsiae and its biofilm by scanning electron microscopy (SEM) using a protocol that faithfully preserved the biofilm architecture and allowed an investigation at very high magnifications (order of nanometers) and with the appropriate resolution. To accomplish this task, we analyzed Streptococcus mutans' biofilm by conventional SEM and VP-SEM protocols, in addition, we developed an original procedure, named OsO4-RR-TA-IL, which avoids dehydration, drying and sputter coating. This innovative protocol allowed high-resolution and high-magnification imaging (from 10000× to 35000×) in high-vacuum and high-voltage conditions. After comparing three methods, we chose OsO4-RR-TA-IL to investigate S. wiggsiae. It appeared as a fusiform elongated bacterium, without surface specialization, arranged in clusters and submerged in a rich biofilm matrix, which showed a well-developed micro-canalicular system. Our results provide the basis for the development of innovative strategies to quantify the effects of different treatments, in order to establish the best option to counteract ECC in pediatric patients.
Keywords: Scardovia wiggsiae; Streptococcus mutans scanning electron microscopy; biofilm; early childhood caries; microbiota; pediatric dentistry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Berg J.H., Slayton R.L. Early Childhood Oral Health. 2nd ed. John Wiley & Sons, Inc.; Hoboken, NJ, USA: 2015.
-
- Douglass J.M., Douglass A.B., Silk H.J. A practical guide to infant oral health. Am. Fam. Physician. 2004;70:2113–2120. - PubMed
-
- Martins-Junior P.A., Vieira-Andrade R.G., Correa-Faria P., Oliveira-Ferreira F., Marques L.S., Ramos-Jorge M.L. Impact of early childhood caries on the oral health-related quality of life of preschool children and their parents. Caries Res. 2013;47:211–218. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

