PARP Inhibitors in the Treatment of Early Breast Cancer: The Step Beyond?
- PMID: 32471249
- PMCID: PMC7352970
- DOI: 10.3390/cancers12061378
PARP Inhibitors in the Treatment of Early Breast Cancer: The Step Beyond?
Abstract
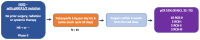
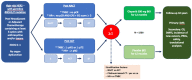
Exquisitely exploiting defects in homologous recombination process, poly(ADP-ribose) polymerase (PARP) inhibitors have recently emerged as a promising class of therapeutics in human epidermal growth factor receptor 2 (HER2)-negative advanced breast cancer with germline breast cancer 1 (BRCA1) or breast cancer 2 (BRCA2) mutations (gBRCA1/2m). In this setting, PARP inhibitors, either as single agent or in combination with platinum-based chemotherapy, significantly increased progression-free survival, as compared to conventional chemotherapy. Accordingly, further therapeutic advances are expected at an earlier stage of the disease. In the neoadjuvant setting, veliparib failed to increase the pathological complete response rate when added to a carboplatin-based regimen, in unselected triple-negative breast cancer patients. Similarly, when administered before anthracycline-cyclophosphamide, the neoadjuvant olaparib-paclitaxel combination was not superior to carboplatin-paclitaxel, in patients with HER2-negative breast cancer and BRCA1/2 mutation, or homologous recombination defect. Yet, neoadjuvant talazoparib, administered as a single-agent in patients with HER2-negative breast cancer and germline BRCA1/2 mutation, achieved an impressive pathological complete response rate of nearly 50%. In the adjuvant setting, the results from the OlympiA phase III study, evaluating adjuvant olaparib in HER2-negative early breast cancer and germline BRCA1/2 mutations, are eagerly awaited. Ongoing trials should clarify whether PARP inhibitors might improve outcome when administered in the adjuvant or neoadjuvant setting in early breast cancer patients with BRCA1/2 mutation or homologous recombination defect.
Keywords: BRCA; PARP inhibitors; early breast cancer.
Conflict of interest statement
A.G. reports travel expenses, accommodation, and meeting registration from Astra Zeneca, Pfizer, Roche, and Novartis. A.B. and F.B. declare no conflict of interest.
Figures

References
-
- Kuchenbaecker K.B., Hopper J.L., Barnes D.R., Phillips K.-A., Mooij T.M., Roos-Blom M.-J., Jervis S., van Leeuwen F.E., Milne R.L., Andrieu N., et al. Risks of breast, ovarian, and contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. JAMA. 2017;317:2402–2416. doi: 10.1001/jama.2017.7112. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

