The Proliferation and Differentiation of Adipose-Derived Stem Cells in Neovascularization and Angiogenesis
- PMID: 32471255
- PMCID: PMC7312564
- DOI: 10.3390/ijms21113790
The Proliferation and Differentiation of Adipose-Derived Stem Cells in Neovascularization and Angiogenesis
Abstract
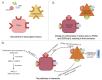
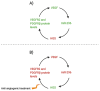
Neovascularization and angiogenesis are vital processes in the repair of damaged tissue, creating new blood vessel networks and increasing oxygen and nutrient supply for regeneration. The importance of Adipose-derived Mesenchymal Stem Cells (ASCs) contained in the adipose tissue surrounding blood vessel networks to these processes remains unknown and the exact mechanisms responsible for directing adipogenic cell fate remain to be discovered. As adipose tissue contains a heterogenous population of partially differentiated cells of adipocyte lineage; tissue repair, angiogenesis and neovascularization may be closely linked to the function of ASCs in a complex relationship. This review aims to investigate the link between ASCs and angiogenesis/neovascularization, with references to current studies. The molecular mechanisms of these processes, as well as ASC differentiation and proliferation are described in detail. ASCs may differentiate into endothelial cells during neovascularization; however, recent clinical trials have suggested that ASCs may also stimulate angiogenesis and neovascularization indirectly through the release of paracrine factors.
Keywords: adipose; angiogenesis; differentiation; stem; vascularization.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

