Endothelial TRPV1 as an Emerging Molecular Target to Promote Therapeutic Angiogenesis
- PMID: 32471282
- PMCID: PMC7349285
- DOI: 10.3390/cells9061341
Endothelial TRPV1 as an Emerging Molecular Target to Promote Therapeutic Angiogenesis
Abstract
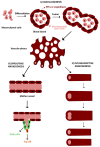
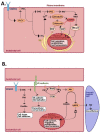
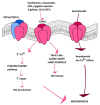
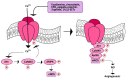
Therapeutic angiogenesis represents an emerging strategy to treat ischemic diseases by stimulating blood vessel growth to rescue local blood perfusion. Therefore, injured microvasculature may be repaired by stimulating resident endothelial cells or circulating endothelial colony forming cells (ECFCs) or by autologous cell-based therapy. Endothelial Ca2+ signals represent a crucial player in angiogenesis and vasculogenesis; indeed, several angiogenic stimuli induce neovessel formation through an increase in intracellular Ca2+ concentration. Several members of the Transient Receptor Potential (TRP) channel superfamily are expressed and mediate Ca2+-dependent functions in vascular endothelial cells and in ECFCs, the only known truly endothelial precursor. TRP Vanilloid 1 (TRPV1), a polymodal cation channel, is emerging as an important player in endothelial cell migration, proliferation, and tubulogenesis, through the integration of several chemical stimuli. Herein, we first summarize TRPV1 structure and gating mechanisms. Next, we illustrate the physiological roles of TRPV1 in vascular endothelium, focusing our attention on how endothelial TRPV1 promotes angiogenesis. In particular, we describe a recent strategy to stimulate TRPV1-mediated pro-angiogenic activity in ECFCs, in the presence of a photosensitive conjugated polymer. Taken together, these observations suggest that TRPV1 represents a useful target in the treatment of ischemic diseases.
Keywords: Ca2+ signaling; TRPV1; endothelial colony forming cells; erythropoietin; evodiamine; organic semiconductors; photostimulation; simvastatin; therapeutic angiogenesis; vascular endothelial cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Endothelial Transient Receptor Potential Channels and Vascular Remodeling: Extracellular Ca2 + Entry for Angiogenesis, Arteriogenesis and Vasculogenesis.Front Physiol. 2020 Jan 21;10:1618. doi: 10.3389/fphys.2019.01618. eCollection 2019. Front Physiol. 2020. PMID: 32038296 Free PMC article. Review.
-
Conjugated polymers mediate intracellular Ca2+ signals in circulating endothelial colony forming cells through the reactive oxygen species-dependent activation of Transient Receptor Potential Vanilloid 1 (TRPV1).Cell Calcium. 2022 Jan;101:102502. doi: 10.1016/j.ceca.2021.102502. Epub 2021 Nov 19. Cell Calcium. 2022. PMID: 34896699
-
The essential role of transient receptor potential vanilloid 1 in simvastatin-induced activation of endothelial nitric oxide synthase and angiogenesis.Acta Physiol (Oxf). 2014 Nov;212(3):191-204. doi: 10.1111/apha.12378. Epub 2014 Sep 20. Acta Physiol (Oxf). 2014. PMID: 25183024
-
Manipulating Intracellular Ca2+ Signals to Stimulate Therapeutic Angiogenesis in Cardiovascular Disorders.Curr Pharm Biotechnol. 2018;19(9):686-699. doi: 10.2174/1389201019666180808165309. Curr Pharm Biotechnol. 2018. PMID: 30091405 Review.
-
Therapeutic Potential of Endothelial Colony-Forming Cells in Ischemic Disease: Strategies to Improve their Regenerative Efficacy.Int J Mol Sci. 2020 Oct 7;21(19):7406. doi: 10.3390/ijms21197406. Int J Mol Sci. 2020. PMID: 33036489 Free PMC article. Review.
Cited by
-
Neurodegeneration, memory loss, and dementia: the impact of biological clocks and circadian rhythm.Front Biosci (Landmark Ed). 2021 Sep 30;26(9):614-627. doi: 10.52586/4971. Front Biosci (Landmark Ed). 2021. PMID: 34590471 Free PMC article. Review.
-
Why Multitarget Vasodilatory (Endo)cannabinoids Are Not Effective as Antihypertensive Compounds after Chronic Administration: Comparison of Their Effects on Systemic and Pulmonary Hypertension.Pharmaceuticals (Basel). 2022 Sep 7;15(9):1119. doi: 10.3390/ph15091119. Pharmaceuticals (Basel). 2022. PMID: 36145339 Free PMC article. Review.
-
The Protection of Zinc against Acute Cadmium Exposure: A Morphological and Molecular Study on a BBB In Vitro Model.Cells. 2022 May 15;11(10):1646. doi: 10.3390/cells11101646. Cells. 2022. PMID: 35626683 Free PMC article.
-
Sensory neuron transient receptor potential vanilloid-1 channel regulates angiogenesis through CGRP in vivo.Front Bioeng Biotechnol. 2024 Mar 21;12:1338504. doi: 10.3389/fbioe.2024.1338504. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 38576442 Free PMC article.
-
Dual Role of TRPV1 Channels in Cerebral Stroke: An Exploration from a Mechanistic and Therapeutic Perspective.Mol Neurobiol. 2024 Dec;61(12):10574-10592. doi: 10.1007/s12035-024-04221-5. Epub 2024 May 17. Mol Neurobiol. 2024. PMID: 38760620 Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

