The impact of remission and coexisting migraine on anxiety and depression in cluster headache
- PMID: 32471362
- PMCID: PMC7257141
- DOI: 10.1186/s10194-020-01120-7
The impact of remission and coexisting migraine on anxiety and depression in cluster headache
Abstract
Background: Our aim was to investigate the relationship between coexisting cluster headache (CH) and migraine with anxiety and depression during active cluster bouts, and how symptoms change during remission.
Methods: We analyzed data from 222 consecutive CH patients and 99 age- and sex-matched controls using a prospective multicenter registry. Anxiety or depression was evaluated using the Generalized Anxiety Disorder-7 (GAD-7) or Patient Health Questionnaire-9 (PHQ-9), respectively. Moderate-to-severe anxiety or depression was defined as a score of ≥10 at baseline (during a cluster bout). We assessed for changes in anxiety and depression during CH remission periods.
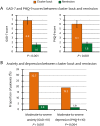
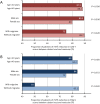
Results: Among the CH patients, the prevalence of moderate-to-severe anxiety and depression was seen in 38.2% and 34.6%, respectively. Compared with controls, CH patients were associated with moderate-to-severe anxiety and depression (multivariable-adjusted odds ratio [aOR] = 7.32, 95% confidence intervals [CI] = 3.35-15.99 and aOR = 4.95, 95% CI = 2.32-10.57, respectively). CH patients with migraine were significantly more likely to have moderate-to-severe anxiety and depression (aOR = 32.53, 95% CI = 6.63-159.64 and aOR = 16.88, 95% CI = 4.16-68.38, respectively), compared to controls without migraine. The GAD-7 and PHQ-9 scores were significantly reduced between cluster bout and remission periods (from 6.8 ± 5.6 to 1.6 ± 2.8; P < 0.001, and from 6.1 ± 5.0 to 1.8 ± 2.4; P < 0.001, respectively).
Conclusions: Our results indicate that CH patients are at increased risk of anxiety and depression, especially in the presence of coexisting migraine. However, the anxiety and depression can improve during remission periods.
Keywords: Anxiety; Cluster headache; Depression; Headache; Migraine.
Conflict of interest statement
Drs. BS Kim, PW Chung, BK Kim, MJ Lee, JW Park, JY Ahn, DW Bae, TJ Song, JH Sohn, K Oh, D Kim, JM Kim, SK Kim, YJ Choi, JM Chung, HS Moon, CS Chung, and KY Park report no conflict of interest.
Dr. Chu was involved as a site investigator for a multicenter trial sponsored by Otsuka Korea, Allergan, Ildong Pharmaceutical Co., LTD, Novartis International AG, and Eli Lilly and Company. He worked an advisory member for Teva, and received lecture honoraria from Allergan Korea, Handok-Teva and Yuyu Pharmaceutical Company in the past 24 months.
Dr. Cho was involved as a site investigator of multicenter trial sponsored Otsuka Korea, Allergan, Ildong Pharmaceutical Co., LTD, Novartis International AG, Eli Lilly and Company. Parexel Korea Co., Ltd., and and received lecture honoraria from Allergan Korea, WhanIn Pharm Co., LTD, Handok-Teva and Yuyu Pharmaceutical Company.
Figures
Similar articles
-
Depression and anxiety in episodic and chronic cluster headache: a pilot study.Headache. 2012 Apr;52(4):600-11. doi: 10.1111/j.1526-4610.2011.02024.x. Epub 2011 Nov 11. Headache. 2012. PMID: 22077836
-
Widespread Hypersensitivity to Pressure Pain in Men With Cluster Headache During Prolonged Remission Is Not Related to the Levels of Depression and Anxiety.Pain Pract. 2020 Feb;20(2):147-153. doi: 10.1111/papr.12839. Epub 2019 Oct 16. Pain Pract. 2020. PMID: 31538698
-
Comorbid and co-occurring conditions in migraine and associated risk of increasing headache pain intensity and headache frequency: results of the migraine in America symptoms and treatment (MAST) study.J Headache Pain. 2020 Mar 2;21(1):23. doi: 10.1186/s10194-020-1084-y. J Headache Pain. 2020. PMID: 32122324 Free PMC article.
-
The psychiatric comorbidities of cluster headache.Curr Pain Headache Rep. 2013 Feb;17(2):313. doi: 10.1007/s11916-012-0313-8. Curr Pain Headache Rep. 2013. PMID: 23296640 Review.
-
[Anxiety and depression in children and adolescents with migraine: a review of the literature].Encephale. 2008 Oct;34(5):504-10. doi: 10.1016/j.encep.2007.08.005. Epub 2007 Dec 26. Encephale. 2008. PMID: 19068340 Review. French.
Cited by
-
Diagnostic Delay and Its Predictors in Cluster Headache.Front Neurol. 2022 Feb 10;13:827734. doi: 10.3389/fneur.2022.827734. eCollection 2022. Front Neurol. 2022. PMID: 35222255 Free PMC article.
-
Urine 5-Hydroxyindoleacetic Acid Negatively Correlates with Migraine Occurrence and Characteristics in the Interictal Phase of Episodic Migraine.Int J Mol Sci. 2024 May 17;25(10):5471. doi: 10.3390/ijms25105471. Int J Mol Sci. 2024. PMID: 38791512 Free PMC article.
-
Inverse association of obesity with bout periodicity in episodic cluster headache: a multicenter cross-sectional study.J Headache Pain. 2025 Jun 20;26(1):144. doi: 10.1186/s10194-025-02061-9. J Headache Pain. 2025. PMID: 40542389 Free PMC article.
-
Phenotype of Cluster Headache: Clinical Variability, Persisting Pain Between Attacks, and Comorbidities-An Observational Cohort Study in 825 Patients.Pain Ther. 2021 Dec;10(2):1121-1137. doi: 10.1007/s40122-021-00267-8. Epub 2021 May 4. Pain Ther. 2021. PMID: 33945123 Free PMC article.
-
The relation of cluster headache to alexithymia, depression, and anxiety.Arq Neuropsiquiatr. 2024 Nov;82(11):1-5. doi: 10.1055/s-0044-1791516. Epub 2024 Nov 3. Arq Neuropsiquiatr. 2024. PMID: 39489152 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

