Induced pluripotent stem cell-based disease modeling identifies ligand-induced decay of megalin as a cause of Donnai-Barrow syndrome
- PMID: 32471643
- PMCID: PMC7322522
- DOI: 10.1016/j.kint.2020.02.021
Induced pluripotent stem cell-based disease modeling identifies ligand-induced decay of megalin as a cause of Donnai-Barrow syndrome
Erratum in
-
Corrigendum to Flemming J, Marczenke M, Rudolph I-M, et al. Induced pluripotent stem cell-based disease modeling identifies ligand-induced decay of megalin as a cause of Donnai-Barrow syndrome. Kidney Int. 2020;98:159-167.Kidney Int. 2021 Aug;100(2):482. doi: 10.1016/j.kint.2021.06.014. Kidney Int. 2021. PMID: 34294211 Free PMC article. No abstract available.
Abstract
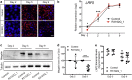
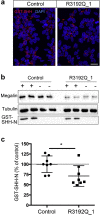
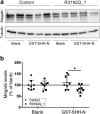
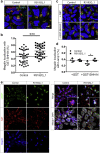
Donnai-Barrow syndrome (DBS) is an autosomal-recessive disorder characterized by multiple pathologies including malformation of forebrain and eyes, as well as resorption defects of the kidney proximal tubule. The underlying cause of DBS are mutations in LRP2, encoding the multifunctional endocytic receptor megalin. Here, we identified a unique missense mutation R3192Q of LRP2 in an affected family that may provide novel insights into the molecular causes of receptor dysfunction in the kidney proximal tubule and other tissues affected in DBS. Using patient-derived induced pluripotent stem cell lines we generated neuroepithelial and kidney cell types as models of the disease. Using these cell models, we documented the inability of megalin R3192Q to properly discharge ligand and ligand-induced receptor decay in lysosomes. Thus, mutant receptors are aberrantly targeted to lysosomes for catabolism, essentially depleting megalin in the presence of ligand in this affected family.
Keywords: endocytosis; low-molecular-weight proteinuria; proximal tubule dysfunction; renal Fanconi syndrome.
Copyright © 2020 International Society of Nephrology. Published by Elsevier Inc. All rights reserved.
Figures



References
-
- Christ A., Christa A., Klippert J. LRP2 acts as SHH clearance receptor to protect the retinal margin from mitogenic stimuli. Dev Cell. 2015;35:36–48. - PubMed
-
- Christ A., Christa A., Kur E. LRP2 is an auxiliary SHH receptor required to condition the forebrain ventral midline for inductive signals. Dev Cell. 2012;22:268–278. - PubMed
-
- Cases O., Obry A., Ben-Yacoub S. Impaired vitreous composition and retinal pigment epithelium function in the FoxG1::LRP2 myopic mice. Biochim Biophys Acta Mol Basis Dis. 2017;1863:1242–1254. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources

