Functional Connectome Analyses Reveal the Human Olfactory Network Organization
- PMID: 32471848
- PMCID: PMC7418535
- DOI: 10.1523/ENEURO.0551-19.2020
Functional Connectome Analyses Reveal the Human Olfactory Network Organization
Abstract
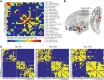
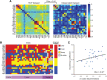
The olfactory system is uniquely heterogeneous, performing multifaceted functions (beyond basic sensory processing) across diverse, widely distributed neural substrates. While knowledge of human olfaction continues to grow, it remains unclear how the olfactory network is organized to serve this unique set of functions. Leveraging a large and high-quality resting-state functional magnetic resonance imaging (rs-fMRI) dataset of nearly 900 participants from the Human Connectome Project (HCP), we identified a human olfactory network encompassing cortical and subcortical regions across the temporal and frontal lobes. Highlighting its reliability and generalizability, the connectivity matrix of this olfactory network mapped closely onto that extracted from an independent rs-fMRI dataset. Graph theoretical analysis further explicated the organizational principles of the network. The olfactory network exhibits a modular composition of three (i.e., the sensory, limbic, and frontal) subnetworks and demonstrates strong small-world properties, high in both global integration and local segregation (i.e., circuit specialization). This network organization thus ensures the segregation of local circuits, which are nonetheless integrated via connecting hubs [i.e., amygdala (AMY) and anterior insula (INSa)], thereby enabling the specialized, yet integrative, functions of olfaction. In particular, the degree of local segregation positively predicted olfactory discrimination performance in the independent sample, which we infer as a functional advantage of the network organization. In sum, an olfactory functional network has been identified through the large HCP dataset, affording a representative template of the human olfactory functional neuroanatomy. Importantly, the topological analysis of the olfactory network provides network-level insights into the remarkable functional specialization and spatial segregation of the olfactory system.
Keywords: functional neuroanatomy; functional segregation; functional specialization; graph theory; network; olfaction.
Copyright © 2020 Arnold et al.
Figures


References
-
- Ash J, Newth D (2007) Optimizing complex networks for resilience against cascading failure. Phys A Stat Mech its Appl 380:673–683. 10.1016/j.physa.2006.12.058 - DOI
-
- Bastian M, Heymann S, Jacomy M (2009) Gephi: an open source software for exploring and manipulating networks. Third International AAAI Conference on Weblogs and Social Media, May 17–20, San Jose, California.
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
