Quantitative Proteome Profiling Reveals Cellobiose-Dependent Protein Processing and Export Pathways for the Lignocellulolytic Response in Neurospora crassa
- PMID: 32471912
- PMCID: PMC7376555
- DOI: 10.1128/AEM.00653-20
Quantitative Proteome Profiling Reveals Cellobiose-Dependent Protein Processing and Export Pathways for the Lignocellulolytic Response in Neurospora crassa
Abstract
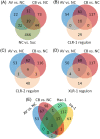
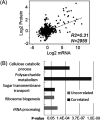
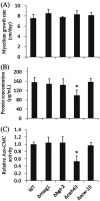
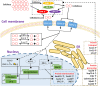
Filamentous fungi are intensively used for producing industrial enzymes, including lignocellulases. Employing insoluble cellulose to induce the production of lignocellulases causes some drawbacks, e.g., a complex fermentation operation, which can be overcome by using soluble inducers such as cellobiose. Here, a triple β-glucosidase mutant of Neurospora crassa, which prevents rapid turnover of cellobiose and thus allows the disaccharide to induce lignocellulases, was applied to profile the proteome responses to cellobiose and cellulose (Avicel). Our results revealed a shared proteomic response to cellobiose and Avicel, whose elements included lignocellulases and cellulolytic product transporters. While the cellulolytic proteins showed a correlated increase in protein and mRNA levels, only a moderate correlation was observed on a proteomic scale between protein and mRNA levels (R2 = 0.31). Ribosome biogenesis and rRNA processing were significantly overrepresented in the protein set with increased protein but unchanged mRNA abundances in response to Avicel. Ribosome biogenesis, as well as protein processing and protein export, was also enriched in the protein set that showed increased abundance in response to cellobiose. NCU05895, a homolog of yeast CWH43, is potentially involved in transferring a glycosylphosphatidylinositol (GPI) anchor to nascent proteins. This protein showed increased abundance but no significant change in mRNA levels. Disruption of CWH43 resulted in a significant decrease in cellulase activities and secreted protein levels in cultures grown on Avicel, suggesting a positive regulatory role for CWH43 in cellulase production. The findings should have an impact on a systems engineering approach for strain improvement for the production of lignocellulases.IMPORTANCE Lignocellulases are important industrial enzymes for sustainable production of biofuels and bio-products. Insoluble cellulose has been commonly used to induce the production of lignocellulases in filamentous fungi, which causes a difficult fermentation operation and enzyme loss due to adsorption to cellulose. The disadvantages can be overcome by using soluble inducers, such as the disaccharide cellobiose. Quantitative proteome profiling of the model filamentous fungus Neurospora crassa revealed cellobiose-dependent pathways for cellulase production, including protein processing and export. A protein (CWH43) potentially involved in protein processing was found to be a positive regulator of lignocellulase production. The cellobiose-dependent mechanisms provide new opportunities to improve the production of lignocellulases in filamentous fungi.
Keywords: Neurospora; cellobiose; cellulase; cellulose; glycosylphosphatidylinositols; protein folding; protein translocation; protein transport; proteomics; ribosome synthesis.
Copyright © 2020 American Society for Microbiology.
Figures
Similar articles
-
Evidence for transceptor function of cellodextrin transporters in Neurospora crassa.J Biol Chem. 2014 Jan 31;289(5):2610-9. doi: 10.1074/jbc.M113.533273. Epub 2013 Dec 16. J Biol Chem. 2014. PMID: 24344125 Free PMC article.
-
Direct cellobiose production from cellulose using sextuple beta-glucosidase gene deletion Neurospora crassa mutants.Enzyme Microb Technol. 2013 Mar 5;52(3):184-9. doi: 10.1016/j.enzmictec.2012.12.010. Epub 2013 Jan 7. Enzyme Microb Technol. 2013. PMID: 23410930
-
Induction of lignocellulose-degrading enzymes in Neurospora crassa by cellodextrins.Proc Natl Acad Sci U S A. 2012 Apr 17;109(16):6012-7. doi: 10.1073/pnas.1118440109. Epub 2012 Apr 2. Proc Natl Acad Sci U S A. 2012. PMID: 22474347 Free PMC article.
-
[Mechanisms and regulation of enzymatic hydrolysis of cellulose in filamentous fungi: classical cases and new models].Rev Iberoam Micol. 2015 Jan-Mar;32(1):1-12. doi: 10.1016/j.riam.2013.10.009. Epub 2014 Mar 7. Rev Iberoam Micol. 2015. PMID: 24607657 Review. Spanish.
-
Biotechnological production of ethanol from renewable resources by Neurospora crassa: an alternative to conventional yeast fermentations?Appl Microbiol Biotechnol. 2013 Feb;97(4):1457-73. doi: 10.1007/s00253-012-4655-2. Epub 2013 Jan 15. Appl Microbiol Biotechnol. 2013. PMID: 23318834 Review.
Cited by
-
Comparative Genomic Analysis Reveals the Functional Traits and Safety Status of Lactic Acid Bacteria Retrieved from Artisanal Cheeses and Raw Sheep Milk.Foods. 2023 Feb 1;12(3):599. doi: 10.3390/foods12030599. Foods. 2023. PMID: 36766127 Free PMC article.
-
Improving the quality of Napier grass silage with pyroligneous acid: Fermentation, aerobic stability, and microbial communities.Front Microbiol. 2022 Nov 14;13:1034198. doi: 10.3389/fmicb.2022.1034198. eCollection 2022. Front Microbiol. 2022. PMID: 36523820 Free PMC article.
-
The Sordariomycetes: an expanding resource with Big Data for mining in evolutionary genomics and transcriptomics.Front Fungal Biol. 2023 Jun 30;4:1214537. doi: 10.3389/ffunb.2023.1214537. eCollection 2023. Front Fungal Biol. 2023. PMID: 37746130 Free PMC article. Review.
-
Exploring the heterogeneity of osteosarcoma cell characteristics and metabolic states and their association with clinical prognosis.Front Immunol. 2024 Dec 6;15:1507476. doi: 10.3389/fimmu.2024.1507476. eCollection 2024. Front Immunol. 2024. PMID: 39712023 Free PMC article.
References
-
- Sukumaran RK, Singhania RR, Pandey A. 2005. Microbial cellulases—production, applications and challenges. J Sci Ind Res 64:832–844.
-
- Gabelle JC, Jourdier E, Licht RB, Ben Chaabane F, Henaut I, Morchain J, Augier F. 2012. Impact of rheology on the mass transfer coefficient during the growth phase of Trichoderma reesei in stirred bioreactors. Chem Eng Sci 75:408–417. doi:10.1016/j.ces.2012.03.053. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

