Contribution of Complex I NADH Dehydrogenase to Respiratory Energy Coupling in Glucose-Grown Cultures of Ogataea parapolymorpha
- PMID: 32471916
- PMCID: PMC7376551
- DOI: 10.1128/AEM.00678-20
Contribution of Complex I NADH Dehydrogenase to Respiratory Energy Coupling in Glucose-Grown Cultures of Ogataea parapolymorpha
Abstract
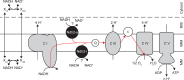
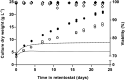
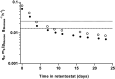
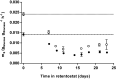
The thermotolerant yeast Ogataea parapolymorpha (formerly Hansenula polymorpha) is an industrially relevant production host that exhibits a fully respiratory sugar metabolism in aerobic batch cultures. NADH-derived electrons can enter its mitochondrial respiratory chain either via a proton-translocating complex I NADH-dehydrogenase or via three putative alternative NADH dehydrogenases. This respiratory entry point affects the amount of ATP produced per NADH/O2 consumed and therefore impacts the maximum yield of biomass and/or cellular products from a given amount of substrate. To investigate the physiological importance of complex I, a wild-type O. parapolymorpha strain and a congenic complex I-deficient mutant were grown on glucose in aerobic batch, chemostat, and retentostat cultures in bioreactors. In batch cultures, the two strains exhibited a fully respiratory metabolism and showed the same growth rates and biomass yields, indicating that, under these conditions, the contribution of NADH oxidation via complex I was negligible. Both strains also exhibited a respiratory metabolism in glucose-limited chemostat cultures, but the complex I-deficient mutant showed considerably reduced biomass yields on substrate and oxygen, consistent with a lower efficiency of respiratory energy coupling. In glucose-limited retentostat cultures at specific growth rates down to ∼0.001 h-1, both O. parapolymorpha strains showed high viability. Maintenance energy requirements at these extremely low growth rates were approximately 3-fold lower than estimated from faster-growing chemostat cultures, indicating a stringent-response-like behavior. Quantitative transcriptome and proteome analyses indicated condition-dependent expression patterns of complex I subunits and of alternative NADH dehydrogenases that were consistent with physiological observations.IMPORTANCE Since popular microbial cell factories have typically not been selected for efficient respiratory energy coupling, their ATP yields from sugar catabolism are often suboptimal. In aerobic industrial processes, suboptimal energy coupling results in reduced product yields on sugar, increased process costs for oxygen transfer, and volumetric productivity limitations due to limitations in gas transfer and cooling. This study provides insights into the contribution of mechanisms of respiratory energy coupling in the yeast cell factory Ogataea parapolymorpha under different growth conditions and provides a basis for rational improvement of energy coupling in yeast cell factories. Analysis of energy metabolism of O. parapolymorpha at extremely low specific growth rates indicated that this yeast reduces its energy requirements for cellular maintenance under extreme energy limitation. Exploration of the mechanisms for this increased energetic efficiency may contribute to an optimization of the performance of industrial processes with slow-growing eukaryotic cell factories.
Keywords: Hansenula polymorpha; NADH; P/O ratio; bioenergetics; bioreactor; chemostat; proteomics; respiration; retentostat; transcriptomics.
Copyright © 2020 Juergens et al.
Figures



Similar articles
-
Respiratory reoxidation of NADH is a key contributor to high oxygen requirements of oxygen-limited cultures of Ogataea parapolymorpha.FEMS Yeast Res. 2022 Feb 22;22(1):foac007. doi: 10.1093/femsyr/foac007. FEMS Yeast Res. 2022. PMID: 35137036 Free PMC article.
-
Physiological relevance, localization and substrate specificity of the alternative (type II) mitochondrial NADH dehydrogenases of Ogataea parapolymorpha.Front Microbiol. 2024 Dec 12;15:1473869. doi: 10.3389/fmicb.2024.1473869. eCollection 2024. Front Microbiol. 2024. PMID: 39726963 Free PMC article.
-
Pichia pastoris Exhibits High Viability and a Low Maintenance Energy Requirement at Near-Zero Specific Growth Rates.Appl Environ Microbiol. 2016 Jul 15;82(15):4570-4583. doi: 10.1128/AEM.00638-16. Print 2016 Aug 1. Appl Environ Microbiol. 2016. PMID: 27208115 Free PMC article.
-
Alternative respiratory pathways of Escherichia coli: energetics and transcriptional regulation in response to electron acceptors.Biochim Biophys Acta. 1997 Jul 4;1320(3):217-34. doi: 10.1016/s0005-2728(97)00034-0. Biochim Biophys Acta. 1997. PMID: 9230919 Review.
-
Upflow anaerobic sludge blanket reactor--a review.Indian J Environ Health. 2001 Apr;43(2):1-82. Indian J Environ Health. 2001. PMID: 12397675 Review.
Cited by
-
A repackaged CRISPR platform increases homology-directed repair for yeast engineering.Nat Chem Biol. 2022 Jan;18(1):38-46. doi: 10.1038/s41589-021-00893-5. Epub 2021 Oct 28. Nat Chem Biol. 2022. PMID: 34711982
-
Protein production dynamics and physiological adaptation of recombinant Komagataella phaffii at near-zero growth rates.Microb Cell Fact. 2024 Feb 8;23(1):43. doi: 10.1186/s12934-024-02314-3. Microb Cell Fact. 2024. PMID: 38331812 Free PMC article.
-
Specific growth rates and growth stoichiometries of Saccharomycotina yeasts on ethanol as sole carbon and energy substrate.FEMS Yeast Res. 2024 Jan 9;24:foae037. doi: 10.1093/femsyr/foae037. FEMS Yeast Res. 2024. PMID: 39656626 Free PMC article.
-
Quantitative physiology and biomass composition of Cyberlindnera jadinii in ethanol-grown cultures.Biotechnol Biofuels Bioprod. 2024 Dec 4;17(1):142. doi: 10.1186/s13068-024-02585-3. Biotechnol Biofuels Bioprod. 2024. PMID: 39633424 Free PMC article.
-
Yeast metabolic innovations emerged via expanded metabolic network and gene positive selection.Mol Syst Biol. 2021 Oct;17(10):e10427. doi: 10.15252/msb.202110427. Mol Syst Biol. 2021. PMID: 34676984 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

