Specific Root Exudate Compounds Sensed by Dedicated Chemoreceptors Shape Azospirillum brasilense Chemotaxis in the Rhizosphere
- PMID: 32471917
- PMCID: PMC7376562
- DOI: 10.1128/AEM.01026-20
Specific Root Exudate Compounds Sensed by Dedicated Chemoreceptors Shape Azospirillum brasilense Chemotaxis in the Rhizosphere
Abstract
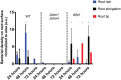
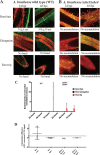
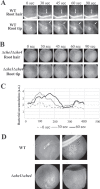
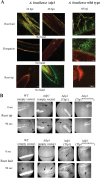
Plant roots shape the rhizosphere community by secreting compounds that recruit diverse bacteria. Colonization of various plant roots by the motile alphaproteobacterium Azospirillum brasilense causes increased plant growth, root volume, and crop yield. Bacterial chemotaxis in this and other motile soil bacteria is critical for competitive colonization of the root surfaces. The role of chemotaxis in root surface colonization has previously been established by endpoint analyses of bacterial colonization levels detected a few hours to days after inoculation. More recently, microfluidic devices have been used to study plant-microbe interactions, but these devices are size limited. Here, we use a novel slide-in chamber that allows real-time monitoring of plant-microbe interactions using agriculturally relevant seedlings to characterize how bacterial chemotaxis mediates plant root surface colonization during the association of A. brasilense with Triticum aestivum (wheat) and Medicago sativa (alfalfa) seedlings. We track A. brasilense accumulation in the rhizosphere and on the root surfaces of wheat and alfalfa. A. brasilense motile cells display distinct chemotaxis behaviors in different regions of the roots, including attractant and repellent responses that ultimately drive surface colonization patterns. We also combine these observations with real-time analyses of behaviors of wild-type and mutant strains to link chemotaxis responses to distinct chemicals identified in root exudates to specific chemoreceptors that together explain the chemotactic response of motile cells in different regions of the roots. Furthermore, the bacterial second messenger c-di-GMP modulates these chemotaxis responses. Together, these findings illustrate dynamic bacterial chemotaxis responses to rhizosphere gradients that guide root surface colonization.IMPORTANCE Plant root exudates play critical roles in shaping rhizosphere microbial communities, and the ability of motile bacteria to respond to these gradients mediates competitive colonization of root surfaces. Root exudates are complex chemical mixtures that are spatially and temporally dynamic. Identifying the exact chemical(s) that mediates the recruitment of soil bacteria to specific regions of the roots is thus challenging. Here, we connect patterns of bacterial chemotaxis responses and sensing by chemoreceptors to chemicals found in root exudate gradients and identify key chemical signals that shape root surface colonization in different plants and regions of the roots.
Keywords: Azospirillum; chemotaxis; rhizosphere; root exudates.
Copyright © 2020 American Society for Microbiology.
Figures



References
-
- Hinsinger P, Bengough AG, Vetterlein D, Young IM. 2009. Rhizosphere: biophysics, biogeochemistry and ecological relevance. Plant Soil 321:117–152. doi:10.1007/s11104-008-9885-9. - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

