The neuropeptide substance P regulates aldosterone secretion in human adrenals
- PMID: 32471973
- PMCID: PMC7260184
- DOI: 10.1038/s41467-020-16470-8
The neuropeptide substance P regulates aldosterone secretion in human adrenals
Abstract
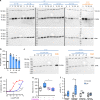
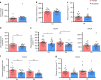
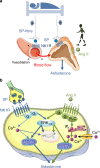
Aldosterone, produced by the adrenals and under the control of plasma angiotensin and potassium levels, regulates hydromineral homeostasis and blood pressure. Here we report that the neuropeptide substance P (SP) released by intraadrenal nerve fibres, stimulates aldosterone secretion via binding to neurokinin type 1 receptors (NK1R) expressed by aldosterone-producing adrenocortical cells. The action of SP is mediated by the extracellular signal-regulated kinase pathway and involves upregulation of steroidogenic enzymes. We also conducted a prospective proof-of-concept, double blind, placebo-controlled clinical trial aimed to investigate the impact of the NK1R antagonist aprepitant on aldosterone secretion in healthy male volunteers (EudraCT: 2008-003367-40, ClinicalTrial.gov: NCT00977223). Participants received during two 7-day treatment periods aprepitant (125 mg on the 1st day and 80 mg during the following days) or placebo in a random order at a 2-week interval. The primary endpoint was plasma aldosterone levels during posture test. Secondary endpoints included basal aldosterone alterations, plasma aldosterone variation during metoclopramide and hypoglycaemia tests, and basal and stimulated alterations of renin, cortisol and ACTH during the three different stimulatory tests. The safety of the treatment was assessed on the basis of serum transaminase measurements on days 4 and 7. All pre-specified endpoints were achieved. Aprepitant decreases aldosterone production by around 30% but does not influence the aldosterone response to upright posture. These results indicate that the autonomic nervous system exerts a direct stimulatory tone on mineralocorticoid synthesis through SP, and thus plays a role in the maintenance of hydromineral homeostasis. This regulatory mechanism may be involved in aldosterone excess syndromes.
Conflict of interest statement
H.L. received payment from MSD Laboratories for lecture unrelated to this study. J.W., C.D., A.-G.L., I.B., H.-G.B., C.D., S.C., F.G. and E.L declare no competing interests.
Figures




References
-
- Staessen J, Lijnen P, Fagard R, Verschueren LJ, Amery A. Rise of plasma aldosterone during long-term captopril treatment. N. Engl. J. Med. 1981;304:1110. - PubMed
-
- Bomback AS, et al. Aldosterone breakthrough during aliskiren, valsartan, and combination (aliskiren + valsartan) therapy. J. Am. Soc. Hypertens. 2012;6:338–345. - PubMed
-
- Schjoedt KJ, Andersen S, Rossing P, Tarnow L, Parving H-H. Aldosterone escape during blockade of the renin-angiotensin-aldosterone system in diabetic nephropathy is associated with enhanced decline in glomerular filtration rate. Diabetologia. 2004;47:1936–1939. - PubMed
-
- Sato A, Saruta T. Aldosterone escape during angiotensin-converting enzyme inhibitor therapy in essential hypertensive patients with left ventricular hypertrophy. J. Int. Med. Res. 2001;29:13–21. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

