CYP17A1 deficient XY mice display susceptibility to atherosclerosis, altered lipidomic profile and atypical sex development
- PMID: 32472014
- PMCID: PMC7260244
- DOI: 10.1038/s41598-020-65601-0
CYP17A1 deficient XY mice display susceptibility to atherosclerosis, altered lipidomic profile and atypical sex development
Abstract
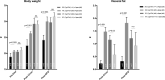
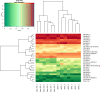
CYP17A1 is a cytochrome P450 enzyme with 17-alpha-hydroxylase and C17,20-lyase activities. CYP17A1 genetic variants are associated with coronary artery disease, myocardial infarction and visceral and subcutaneous fat distribution; however, the underlying pathological mechanisms remain unknown. We aimed to investigate the function of CYP17A1 and its impact on atherosclerosis in mice. At 4-6 months, CYP17A1-deficient mice were viable, with a KO:Het:WT ratio approximating the expected Mendelian ratio of 1:2:1. All Cyp17a1 knockout (KO) mice were phenotypically female; however, 58% were Y chromosome-positive, resembling the phenotype of human CYP17A1 deficiency, leading to 46,XY differences/disorders of sex development (DSD). Both male and female homozygous KO mice were infertile, due to abnormal genital organs. Plasma steroid analyses revealed a complete lack of testosterone in XY-KO mice and marked accumulation of progesterone in XX-KO mice. Elevated corticosterone levels were observed in both XY and XX KO mice. In addition, Cyp17a1 heterozygous mice were also backcrossed onto an Apoe KO atherogenic background and fed a western-type diet (WTD) to study the effects of CYP17A1 on atherosclerosis. Cyp17a1 x Apoe double KO XY mice developed more atherosclerotic lesions than Apoe KO male controls, regardless of diet (standard or WTD). Increased atherosclerosis in CYP17A1 XY KO mice lacking testosterone was associated with altered lipid profiles. In mice, CYP17A1 deficiency interferes with sex differentiation. Our data also demonstrate its key role in lipidomic profile, and as a risk factor in the pathogenesis of atherosclerosis.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Mozaffarian D, et al. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation. 2016;133:e38–360. - PubMed
-
- Erdmann J, Kessler T, Munoz Venegas L, Schunkert H. A decade of genome-wide association studies for coronary artery disease: the challenges ahead. Cardiovasc Res. 2018;114:1241–1257. - PubMed
-
- Hotta K, et al. Genetic variations in the CYP17A1 and NT5C2 genes are associated with a reduction in visceral and subcutaneous fat areas in Japanese women. J Hum Genet. 2012;57:46–51. - PubMed
-
- Pang SY, et al. Worldwide experience in newborn screening for classical congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Pediatrics. 1988;81:866–74. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

