Chemical and Microstructural Characterization of pH and [Ca2+] Dependent Sol-Gel Transitions in Mucin Biopolymer
- PMID: 32472040
- PMCID: PMC7260187
- DOI: 10.1038/s41598-020-65392-4
Chemical and Microstructural Characterization of pH and [Ca2+] Dependent Sol-Gel Transitions in Mucin Biopolymer
Abstract
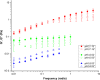
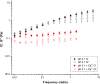
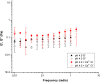
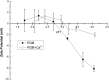
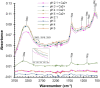
Mucus is responsible for controlling transport and barrier function in biological systems, and its properties can be significantly affected by compositional and environmental changes. In this study, the impacts of pH and CaCl2 were examined on the solution-to-gel transition of mucin, the primary structural component of mucus. Microscale structural changes were correlated with macroscale viscoelastic behavior as a function of pH and calcium addition using rheology, dynamic light scattering, zeta potential, surface tension, and FTIR spectroscopic characterization. Mucin solutions transitioned from solution to gel behavior between pH 4-5 and correspondingly displayed a more than ten-fold increase in viscoelastic moduli. Addition of CaCl2 increased the sol-gel transition pH value to ca. 6, with a twofold increase in loss moduli at low frequencies and ten-fold increase in storage modulus. Changing the ionic conditions-specifically [H+] and [Ca2+] -modulated the sol-gel transition pH, isoelectric point, and viscoelastic properties due to reversible conformational changes with mucin forming a network structure via non-covalent cross-links between mucin chains.
Conflict of interest statement
The authors declare no competing interests.
Figures





Similar articles
-
Rheology of gastric mucin exhibits a pH-dependent sol-gel transition.Biomacromolecules. 2007 May;8(5):1580-6. doi: 10.1021/bm0609691. Epub 2007 Apr 3. Biomacromolecules. 2007. PMID: 17402780
-
Viscoelastic properties of human tracheobronchial mucin in aqueous solution.Biopolymers. 1995 Feb;35(2):149-59. doi: 10.1002/bip.360350203. Biopolymers. 1995. PMID: 7696561
-
pH-dependent conformational change of gastric mucin leads to sol-gel transition.Biophys J. 1999 Mar;76(3):1250-8. doi: 10.1016/S0006-3495(99)77288-7. Biophys J. 1999. PMID: 10049309 Free PMC article.
-
Changes in cervical mucus that prevent penetration by spermatozoa.Symp Soc Exp Biol. 1989;43:325-36. Symp Soc Exp Biol. 1989. PMID: 2701482 Review.
-
Characterization of polyelectrolyte features in polysaccharide systems and mucin.Adv Colloid Interface Sci. 2010 Jul 12;158(1-2):108-18. doi: 10.1016/j.cis.2009.05.003. Epub 2009 May 15. Adv Colloid Interface Sci. 2010. PMID: 19482258 Review.
Cited by
-
Encapsulated salts in velvet worm slime drive its hardening.Sci Rep. 2022 Nov 10;12(1):19261. doi: 10.1038/s41598-022-23523-z. Sci Rep. 2022. PMID: 36357497 Free PMC article.
-
Comprehensive Characterization of the Viscoelastic Properties of Bovine Submaxillary Mucin (BSM) Hydrogels and the Effect of Additives.Biomacromolecules. 2024 Jul 8;25(7):4014-4029. doi: 10.1021/acs.biomac.4c00153. Epub 2024 Jun 4. Biomacromolecules. 2024. PMID: 38832927 Free PMC article.
-
Multi-Functional Polydopamine-Mucin Hollow Particles Provide Tunable Shell Permeability, ROS Scavenging, Tissue Adhesion, and Lubricity for Biomedical Applications.Small. 2025 Aug;21(34):e2503238. doi: 10.1002/smll.202503238. Epub 2025 Jul 4. Small. 2025. PMID: 40611788 Free PMC article.
-
From nanoparticles to crystals: one-pot programmable biosynthesis of photothermal gold structures and their use for biomedical applications.J Nanobiotechnology. 2022 Nov 16;20(1):482. doi: 10.1186/s12951-022-01680-7. J Nanobiotechnology. 2022. PMID: 36384747 Free PMC article.
-
Biopolymer Drug Delivery Systems for Oromucosal Application: Recent Trends in Pharmaceutical R&D.Int J Mol Sci. 2024 May 14;25(10):5359. doi: 10.3390/ijms25105359. Int J Mol Sci. 2024. PMID: 38791397 Free PMC article. Review.
References
-
- Forstner JF, Forstner GG. Effects of Calcium on Intestinal Mucin: Implications for Cystic Fibrosis. Pediatr. Res. 1976;10:609–613. - PubMed
-
- Khutoryanskiy VV. Advances in Mucoadhesion and Mucoadhesive Polymers. Macromol. Biosci. 2011;11:748–764. - PubMed
-
- Svensson, O. Interactions of Mucins with Biopolymers and Drug Delivery Particles. (2008). at, https://muep.mau.se/bitstream/handle/2043/5930/Olof%20Svensson%20Kappan....
LinkOut - more resources
Full Text Sources
Miscellaneous

