Neoplastic fibrocytes play an essential role in bone marrow fibrosis in Jak2V617F-induced primary myelofibrosis mice
- PMID: 32472085
- PMCID: PMC7862060
- DOI: 10.1038/s41375-020-0880-3
Neoplastic fibrocytes play an essential role in bone marrow fibrosis in Jak2V617F-induced primary myelofibrosis mice
Abstract
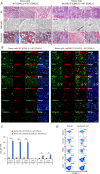
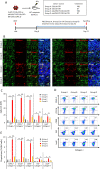
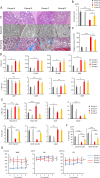
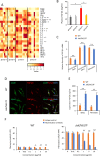
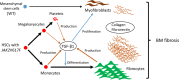
Primary myelofibrosis (PMF) is a myeloproliferative neoplasm (MPN) characterized by clonal myeloproliferation, progressive bone marrow (BM) fibrosis, splenomegaly, and anemia. BM fibrosis was previously thought to be a reactive phenomenon induced by mesenchymal stromal cells that are stimulated by the overproduction of cytokines such as transforming growth factor (TGF)-β1. However, the involvement of neoplastic fibrocytes in BM fibrosis was recently reported. In this study, we showed that the vast majority of collagen- and fibronectin-producing cells in the BM and spleens of Jak2V617F-induced myelofibrosis (MF) mice were fibrocytes derived from neoplastic hematopoietic cells. Neoplastic monocyte depletion eliminated collagen- and fibronectin-producing fibrocytes in BM and spleen, and ameliorated most characteristic MF features in Jak2V617F transgenic mice, including BM fibrosis, anemia, and splenomegaly, while had little effect on the elevated numbers of megakaryocytes and stem cells in BM, and leukothrombocytosis in peripheral blood. TGF-β1, which was produced by hematopoietic cells including fibrocytes, promoted the differentiation of neoplastic monocytes to fibrocytes, and elevated plasma TGF-β1 levels were normalized by monocyte depletion. Collectively, our data suggest that neoplastic fibrocytes are the major contributor to BM fibrosis in PMF, and TGF-β1 is required for their differentiation.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


Similar articles
-
Primary myelofibrosis marrow-derived CD14+/CD34- monocytes induce myelofibrosis-like phenotype in immunodeficient mice and give rise to megakaryocytes.PLoS One. 2019 Sep 30;14(9):e0222912. doi: 10.1371/journal.pone.0222912. eCollection 2019. PLoS One. 2019. PMID: 31569199 Free PMC article.
-
TGF-β1 protein trap AVID200 beneficially affects hematopoiesis and bone marrow fibrosis in myelofibrosis.JCI Insight. 2021 Sep 22;6(18):e145651. doi: 10.1172/jci.insight.145651. JCI Insight. 2021. PMID: 34383713 Free PMC article.
-
Role of neoplastic monocyte-derived fibrocytes in primary myelofibrosis.J Exp Med. 2016 Aug 22;213(9):1723-40. doi: 10.1084/jem.20160283. Epub 2016 Aug 1. J Exp Med. 2016. PMID: 27481130 Free PMC article.
-
Understanding Splenomegaly in Myelofibrosis: Association with Molecular Pathogenesis.Int J Mol Sci. 2018 Mar 18;19(3):898. doi: 10.3390/ijms19030898. Int J Mol Sci. 2018. PMID: 29562644 Free PMC article. Review.
-
Focus on Osteosclerotic Progression in Primary Myelofibrosis.Biomolecules. 2021 Jan 19;11(1):122. doi: 10.3390/biom11010122. Biomolecules. 2021. PMID: 33477816 Free PMC article. Review.
Cited by
-
Circulating CD34+ cells of primary myelofibrosis patients contribute to myeloid-dominant hematopoiesis and bone marrow fibrosis in immunodeficient mice.Int J Hematol. 2022 Feb;115(2):198-207. doi: 10.1007/s12185-021-03239-y. Epub 2021 Nov 13. Int J Hematol. 2022. PMID: 34773575 Free PMC article.
-
Overview of Myeloproliferative Neoplasms: History, Pathogenesis, Diagnostic Criteria, and Complications.Hematol Oncol Clin North Am. 2021 Apr;35(2):159-176. doi: 10.1016/j.hoc.2020.12.001. Epub 2021 Jan 26. Hematol Oncol Clin North Am. 2021. PMID: 33641861 Free PMC article. Review.
-
C-Mannosyl tryptophan is a novel biomarker for thrombocytosis of myeloproliferative neoplasms.Sci Rep. 2024 Aug 14;14(1):18858. doi: 10.1038/s41598-024-69496-z. Sci Rep. 2024. PMID: 39143127 Free PMC article.
-
ASXL1 mutations accelerate bone marrow fibrosis via EGR1-TNFA axis-mediated neoplastic fibrocyte generation in myeloproliferative neoplasms.Haematologica. 2023 May 1;108(5):1359-1373. doi: 10.3324/haematol.2021.280320. Haematologica. 2023. PMID: 36005555 Free PMC article.
-
Dnmt3a is downregulated by Stat5a and mediates G0/G1 arrest by suppressing the miR-17-5p/Cdkn1a axis in Jak2V617F cells.BMC Cancer. 2021 Nov 13;21(1):1213. doi: 10.1186/s12885-021-08915-0. BMC Cancer. 2021. PMID: 34773997 Free PMC article.
References
-
- Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al. WHO classification of tumours of haematopoietic and lymphoid tissues. 4th ed. Lyon: International Agency for Research on Cancer; 2017. pp. 39–53.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

