Mixed Response to Immunotherapy in Patients with Metastatic Melanoma
- PMID: 32472413
- PMCID: PMC7410859
- DOI: 10.1245/s10434-020-08657-6
Mixed Response to Immunotherapy in Patients with Metastatic Melanoma
Abstract
Background: Immunotherapy has improved overall survival in metastatic melanoma. Response to therapy can be difficult to evaluate as the traditionally used RECIST 1.1 criteria do not capture heterogeneous responses. Here we describe the clinical characterization of melanoma patients with a clinically defined mixed response to immunotherapy.
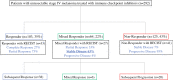
Methods: This was a single institution, retrospective analysis of stage IV melanoma patients who received first-line anti-CTLA-4, anti-PD1, or combination anti-CTLA-4/anti-PD1. Therapy response was assessed via clinical definitions, which consisted of cross-sectional imaging combined with clinical exam. Course of disease, clinicopathological characteristics, and management in patients with a mixed clinical response were analyzed.
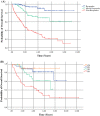
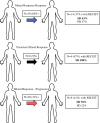
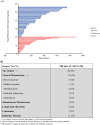
Results: In 292 patients (anti-CTLA4 = 63; anti-PD1 = 148, anti-CTLA4/anti-PD1 = 81), 103 were responders (35%), 64 mixed responders (22%), and 125 patients had progressive disease (43%). Of patients with a mixed response, 56% eventually had response to therapy (mixed response followed by response, MR-R), while 31% progressed on therapy (MR-NR). MR-NR patients had higher median LDH (p < 0.01), 3 or more organ sites with metastases (p < 0.01), and more frequently had M1d disease (p < 0.01). Mixed responders who underwent surgery (n = 20) had a significantly longer mean OS compared to patients who did not undergo surgery (6.9 years, 95% CI 6.2-7.6 vs. 6.0 years, 95% CI 4.6-7.3, p = 0.02).
Discussion: Mixed response to immunotherapy in metastatic melanoma was not uncommon in our cohort (22%). Clinical characteristics associated with progression of disease after initial mixed response included higher LDH, brain metastases, and ≥ 3 organ sites with metastases. Surgical treatment for highly selected patients with a mixed response was associated with improved outcomes.
Conflict of interest statement
Dr. Genevieve Boland reports sponsored research agreements with Takeda Oncology, Olink Proteomics, and Palleon Pharmaceuticals, but these are outside the current work under consideration. She also served as a speaker for Takeda Oncology and Novartis, none of which are in conflict with the manuscript under review. She also served on a scientific advisory board and is on a steering committee with Nektar Therapeutics, again none of which is related to the work submitted. All other authors report no conflicts of interest.
Figures

References
-
- Robert C, Ribas A, Schachter J, Arance A, Grob JJ, Mortier L, et al. Pembrolizumab versus ipilimumab in advanced melanoma (KEYNOTE-006): post hoc 5-year results from an open-label, multicentre, randomised, controlled, phase 3 study. Lancet Oncol. 2019;20(9):1239–1251. doi: 10.1016/S1470-2045(19)30388-2. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

