Ethanol and Cannabinoids Regulate Zebrafish GABAergic Neuron Development and Behavior in a Sonic Hedgehog and Fibroblast Growth Factor-Dependent Mechanism
- PMID: 32472575
- PMCID: PMC7572643
- DOI: 10.1111/acer.14383
Ethanol and Cannabinoids Regulate Zebrafish GABAergic Neuron Development and Behavior in a Sonic Hedgehog and Fibroblast Growth Factor-Dependent Mechanism
Abstract
Background: Ethanol (EtOH) has diverse effects on nervous system development, which includes development and survival of GABAergic neurons in a sonic hedgehog (Shh) and fibroblast growth factor (Fgf)-dependent mechanism. Cannabinoids also function as inhibitors of Shh signaling, raising the possibility that EtOH and cannabinoids may interact to broadly disrupt neuronal function during brain development.
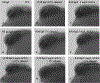
Methods: Zebrafish embryos were exposed to a range of EtOH and/or cannabinoid receptor 1 (CB1R) agonist concentrations at specific developmental stages, in the absence or presence of morpholino oligonucleotides that disrupt shh expression. In situ hybridization was employed to analyze glutamic acid decarboxylase (gad1) gene expression as a marker of GABAergic neuron differentiation, and zebrafish behavior was analyzed using the novel tank diving test as a measure of risk-taking behavior.
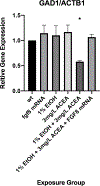
Results: Combined acute subthreshold EtOH and CB1R agonist exposure results in a marked reduction in gad1 mRNA expression in zebrafish forebrain. Consistent with the EtOH and cannabinoid effects on Shh signaling, fgf8 mRNA overexpression rescues the EtOH- and cannabinoid-induced decrease in gad1 gene expression and also prevents the changes in behavior induced by EtOH and cannabinoids.
Conclusions: These studies provide evidence that forebrain GABAergic neuron development and zebrafish risk-taking behavior are sensitive to both EtOH and cannabinoid exposure in a Shh- and Fgf-dependent mechanism, and provide additional evidence that a signaling pathway involving Shh and Fgf crosstalk is a critical target of EtOH and cannabinoids in FASD.
Keywords: Cannabinoid; FASD; Fgf; Shh; gad1.
© 2020 by the Research Society on Alcoholism.
Conflict of interest statement
The authors have no conflicts of interest to report.
Figures




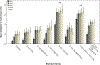
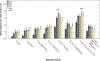


References
-
- Aoto K, Shikata Y, Higashiyama D, Shiota K, Motoyama J (2008) Fetal ethanol exposure activates protein kinase A and impairs Shh expression in prechordal mesendoderm cells in the pathogenesis of holoprosencephaly. Birth Defects Res (Part A) 82: 224–231. - PubMed
-
- Boa-Amponsem O, Zhang C, Mukhopadhyay S, Ardrey A, Cole GJ (2019) Ethanol and cannabinoids interact to alter behavior in a zebrafish fetal alcohol spectrum disorder model. Birth Defects Research 1–14. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

