Fruit water content as an indication of sugar metabolism improves simulation of carbohydrate accumulation in tomato fruit
- PMID: 32472678
- PMCID: PMC7410181
- DOI: 10.1093/jxb/eraa225
Fruit water content as an indication of sugar metabolism improves simulation of carbohydrate accumulation in tomato fruit
Abstract
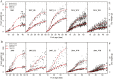
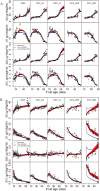
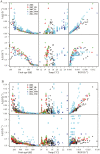
Although fleshy fruit is mainly made up of water, little is known about the impact of its water status on sugar metabolism and its composition. In order to verify whether fruit water status is an important driver of carbohydrate composition in tomato fruit, an adaptation of the SUGAR model proposed previously by M. Génard and M. Souty was used. Two versions of the model, with or without integrating the influence of fruit water content on carbohydrate metabolism, were proposed and then assessed with the data sets from two genotypes, Levovil and Cervil, grown under different conditions. The results showed that, for both genotypes, soluble sugars and starch were better fitted by the model when the effects of water content on carbohydrate metabolism were taken into consideration. Water content might play a regulatory role in the carbon metabolism from sugars to compounds other than sugars and starch in Cervil fruit, and from sugars to starch in Levovil fruit. While water content influences tomato fruit carbohydrate concentrations by both metabolism and dilution/dehydration effects in the early developmental stage, it is mainly by dilution/dehydration effects in the late stage. The possible mechanisms underlying the effect of the fruit water content on carbohydrate metabolism are also discussed.
Keywords: Solanum lycopersicum; Carbohydrate metabolism; genotype×environment interaction; modelling; soluble sugars; starch; water status.
© The Author(s) 2020. Published by Oxford University Press on behalf of the Society for Experimental Biology.
Figures




References
-
- Adams S, Cockshull K, Cave C. 2001. Effect of temperature on the growth and development of tomato fruits. Annals of Botany 88, 869–877.
-
- Baldwin E, Scott J, Einstein M, Malundo T, Carr B, Shewfelt R, Tandon K. 1998. Relationship between sensory and instrumental analysis for tomato flavor. Journal of the American Society for Horticultural Science 123, 906–915.
-
- Balibrea ME, Martínez-Andújar C, Cuartero J, Bolarín MC, Pérez-Alfocea F. 2006. The high fruit soluble sugar content in wild Lycopersicon species and their hybrids with cultivars depends on sucrose import during ripening rather than on sucrose metabolism. Functional Plant Biology 33, 279–288. - PubMed
-
- Beckles DM. 2012. Factors affecting the postharvest soluble solids and sugar content of tomato (Solanum lycopersicum L.) fruit. Postharvest Biology and Technology 63, 129–140.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

