The Perinuclear ER Scales Nuclear Size Independently of Cell Size in Early Embryos
- PMID: 32473090
- PMCID: PMC7423768
- DOI: 10.1016/j.devcel.2020.05.003
The Perinuclear ER Scales Nuclear Size Independently of Cell Size in Early Embryos
Abstract
Nuclear size plays pivotal roles in gene expression, embryo development, and disease. A central hypothesis in organisms ranging from yeast to vertebrates is that nuclear size scales to cell size. This implies that nuclei may reach steady-state sizes set by limiting cytoplasmic pools of size-regulating components. By monitoring nuclear dynamics in early sea urchin embryos, we found that nuclei undergo substantial growth in each interphase, reaching a maximal size prior to mitosis that declined steadily over the course of development. Manipulations of cytoplasmic volume through multiple chemical and physical means ruled out cell size as a major determinant of nuclear size and growth. Rather, our data suggest that the perinuclear endoplasmic reticulum, accumulated through dynein activity, serves as a limiting membrane pool that sets nuclear surface growth rate. Partitioning of this local pool at each cell division modulates nuclear growth kinetics and dictates size scaling throughout early development.
Keywords: cell size; embryonic development; nuclear size scaling; nuclear-to-cytoplasmic ratio; nucleus; paracentrotus lividus sea urchins; perinuclear endoplasmic reticulum; xenopus laevis frogs.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests The authors declare no competing interests.
Figures


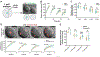


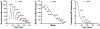

Comment in
-
Nuclear sizER in Early Development.Dev Cell. 2020 Aug 10;54(3):297-298. doi: 10.1016/j.devcel.2020.06.037. Dev Cell. 2020. PMID: 32781022
References
-
- Anderson DJ, and Hetzer MW (2007). Nuclear envelope formation by chromatin-mediated reorganization of the endoplasmic reticulum. Nat Cell Biol 9, 1160–6. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

