Acetyl-CoA Derived from Hepatic Peroxisomal β-Oxidation Inhibits Autophagy and Promotes Steatosis via mTORC1 Activation
- PMID: 32473093
- PMCID: PMC7335356
- DOI: 10.1016/j.molcel.2020.05.007
Acetyl-CoA Derived from Hepatic Peroxisomal β-Oxidation Inhibits Autophagy and Promotes Steatosis via mTORC1 Activation
Abstract
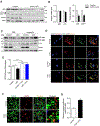
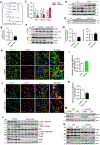
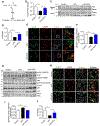
Autophagy is activated by prolonged fasting but cannot overcome the ensuing hepatic lipid overload, resulting in fatty liver. Here, we describe a peroxisome-lysosome metabolic link that restricts autophagic degradation of lipids. Acyl-CoA oxidase 1 (Acox1), the enzyme that catalyzes the first step in peroxisomal β-oxidation, is enriched in liver and further increases with fasting or high-fat diet (HFD). Liver-specific Acox1 knockout (Acox1-LKO) protected mice against hepatic steatosis caused by starvation or HFD due to induction of autophagic degradation of lipid droplets. Hepatic Acox1 deficiency markedly lowered total cytosolic acetyl-CoA levels, which led to decreased Raptor acetylation and reduced lysosomal localization of mTOR, resulting in impaired activation of mTORC1, a central regulator of autophagy. Dichloroacetic acid treatment elevated acetyl-CoA levels, restored mTORC1 activation, inhibited autophagy, and increased hepatic triglycerides in Acox1-LKO mice. These results identify peroxisome-derived acetyl-CoA as a key metabolic regulator of autophagy that controls hepatic lipid homeostasis.
Keywords: Acox1; Autophagy; Lipid metabolism; NAFLD; Raptor; fatty acid oxidation; lipophagy; mTOR; peroxisomes.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures
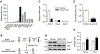


References
-
- Chu BB, Liao YC, Qi W, Xie C, Du X, Wang J, Yang H, Miao HH, Li BL, and Song BL (2015). Cholesterol transport through lysosome-peroxisome membrane contacts. Cell 161, 291–306. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

