Mouse gastrulation: Coordination of tissue patterning, specification and diversification of cell fate
- PMID: 32473204
- PMCID: PMC7534585
- DOI: 10.1016/j.mod.2020.103617
Mouse gastrulation: Coordination of tissue patterning, specification and diversification of cell fate
Abstract
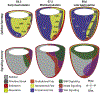
During mouse embryonic development a mass of pluripotent epiblast tissue is transformed during gastrulation to generate the three definitive germ layers: endoderm, mesoderm, and ectoderm. During gastrulation, a spatiotemporally controlled sequence of events results in the generation of organ progenitors and positions them in a stereotypical fashion throughout the embryo. Key to the correct specification and differentiation of these cell fates is the establishment of an axial coordinate system along with the integration of multiple signals by individual epiblast cells to produce distinct outcomes. These signaling domains evolve as the anterior-posterior axis is established and the embryo grows in size. Gastrulation is initiated at the posteriorly positioned primitive streak, from which nascent mesoderm and endoderm progenitors ingress and begin to diversify. Advances in technology have facilitated the elaboration of landmark findings that originally described the epiblast fate map and signaling pathways required to execute those fates. Here we will discuss the current state of the field and reflect on how our understanding has shifted in recent years.
Keywords: Axis patterning; Cell fate specification; Gastrulation; Mouse development; Organogenesis.
Copyright © 2020 Elsevier B.V. All rights reserved.
Figures
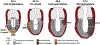
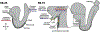
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

