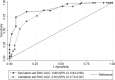
Derivation and validation of a scoring system to assess pre-test probability of being COVID-19 positive
- PMID: 32473233
- PMCID: PMC7255159
- DOI: 10.1016/j.jinf.2020.05.044
Derivation and validation of a scoring system to assess pre-test probability of being COVID-19 positive
Keywords: COVID-19; Diagnosis; SARS-CoV-2; Score.
Conflict of interest statement
Declaration of Competing Interest None.
Figures
Comment on
-
Lymphopenic community acquired pneumonia as signature of severe COVID-19 infection.J Infect. 2020 May;80(5):e23-e24. doi: 10.1016/j.jinf.2020.02.029. Epub 2020 Mar 5. J Infect. 2020. PMID: 32145214 Free PMC article. No abstract available.
References
-
- Kokkinakis I, Selby K, Favrat B, Genton B, Cornuz J. Performance du frottis nasopharyngé-PCR pour le diagnostic du Covid-19 - Recommandations pratiques sur la base des premières données scientifiques [Covid-19 diagnosis: clinical recommendations and performance of nasopharyngeal swab-PCR] Rev Med Suisse. 2020;16(689):699–701. - PubMed
-
- Centers for Disease Control and Prevention Interim Guidelines for Collecting, Handling, and Testing Clinical Specimens from Persons Under Investigation (PUIs) for Coronavirus Disease 2019 (COVID-19) 2020;14 https://www.cdc.gov/coronavirus/2019-nCoV/lab/guidelines-clinical-specim... February. (Accessed on March 15, 2020)
-
- Interim guidance. WHO; 2020. Laboratory testing for 2019 novel coronavirus (2019-nCoV) in suspected human cases.https://www.who.int/publications-detail/laboratory-testing-for-2019-nove... 19 MarchAvailable at.
-
- Sullivan LM1, Massaro JM, D'Agostino RB., Sr Presentation of multivariate data for clinical use: The Framingham Study risk score functions. Stat Med. 2004;23(10):1631–1660. May 30. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous