Discovery of the FDA-approved drugs bexarotene, cetilistat, diiodohydroxyquinoline, and abiraterone as potential COVID-19 treatments with a robust two-tier screening system
- PMID: 32473310
- PMCID: PMC7254006
- DOI: 10.1016/j.phrs.2020.104960
Discovery of the FDA-approved drugs bexarotene, cetilistat, diiodohydroxyquinoline, and abiraterone as potential COVID-19 treatments with a robust two-tier screening system
Abstract
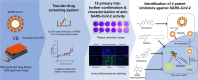
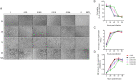
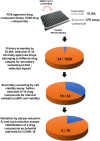
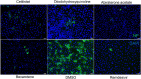
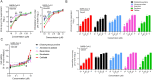
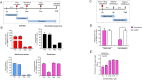
Coronavirus Disease 2019 (COVID-19) caused by the emerging severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is associated with a crude case fatality rate of about 0.5-10 % depending on locality. A few clinically approved drugs, such as remdesivir, chloroquine, hydroxychloroquine, nafamostat, camostat, and ivermectin, exhibited anti-SARS-CoV-2 activity in vitro and/or in a small number of patients. However, their clinical use may be limited by anti-SARS-CoV-2 50 % maximal effective concentrations (EC50) that exceeded their achievable peak serum concentrations (Cmax), side effects, and/or availability. To find more immediately available COVID-19 antivirals, we established a two-tier drug screening system that combines SARS-CoV-2 enzyme-linked immunosorbent assay and cell viability assay, and applied it to screen a library consisting 1528 FDA-approved drugs. Cetilistat (anti-pancreatic lipase), diiodohydroxyquinoline (anti-parasitic), abiraterone acetate (synthetic androstane steroid), and bexarotene (antineoplastic retinoid) exhibited potent in vitro anti-SARS-CoV-2 activity (EC50 1.13-2.01 μM). Bexarotene demonstrated the highest Cmax:EC50 ratio (1.69) which was higher than those of chloroquine, hydroxychloroquine, and ivermectin. These results demonstrated the efficacy of the two-tier screening system and identified potential COVID-19 treatments which can achieve effective levels if given by inhalation or systemically depending on their pharmacokinetics.
Keywords: Abiraterone; Abiraterone acetate (PubChem CID: 9821849); Antiviral; Bexarotene; Bexarotene (PubChem CID: 82146); COVID-19; Cetilistat; Cetilistat (PubChem CID: 9952916); Coronavirus; Diiodohydroxyquinoline; Diiodohydroxyquinoline (PubChem CID: 3728); Library; SARS-CoV-2; Treatment.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Conflict of interest statement
JFWC has received travel grants from Pfizer Corporation Hong Kong and Astellas Pharma Hong Kong Corporation Limited, and was an invited speaker for Gilead Sciences Hong Kong Limited and Luminex Corporation. The other authors declared no conflict of interests. The funding sources had no role in study design, data collection, analysis or interpretation or writing of the report. The corresponding authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Figures

References
-
- World Health Organization . 2020. Coronavirus Disease 2019 (COVID-19) Situation Report – 77.https://www.who.int/docs/default-source/coronaviruse/situation-reports/2...
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

