Investigation of the antigenicity and protective efficacy of Leishmania promastigote membrane antigens in search of potential diagnostic and vaccine candidates against visceral leishmaniasis
- PMID: 32473634
- PMCID: PMC7260476
- DOI: 10.1186/s13071-020-04138-7
Investigation of the antigenicity and protective efficacy of Leishmania promastigote membrane antigens in search of potential diagnostic and vaccine candidates against visceral leishmaniasis
Abstract
Background: Visceral leishmaniasis (VL), is a parasitic disease that causes serious medical consequences if treatment is delayed. Despite a decline in the number of VL cases in the Indian subcontinent, the commencement of the disease in newer areas continues to be a major concern. Although serological diagnosis mainly by immunochromatographic tests has been found to be effective, a test of cure in different phases of treatment is still desired. Even though a good prophylactic response has been obtained in murine models by a number of vaccine candidates, few have been proposed for human use.
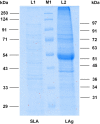
Methods: In this study, nine antigenic components (31, 34, 36, 45, 51, 63, 72, 91 and 97 kDa) of Leishmania promastigote membrane antigens (LAg), were electroeluted and evaluated through ELISA to diagnose and distinguish active VL from one month cured and six months post-treatment patients. Further, to investigate the immunogenicity of electroeluted proteins, human PBMCs of cured VL patients were stimulated with 31, 34, 51, 63, 72 and 91 kDa proteins.
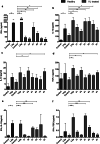
Results: We found that 34 and 51 kDa proteins show 100% sensitivity and specificity with healthy controls and other diseases. After six months post-treatment, antibodies to 72 and 91 kDa antigens show a significant decline to almost normal levels. This suggests that 34 and 51 kDa proteins are efficient in diagnosis, whereas 72 and 91 kDa proteins may be used to monitor treatment outcome. In another assay, 51 and 63 kDa proteins demonstrated maximum ability to upregulate IFN-γ and IL-12 with minimum induction of IL-10 and TGF-β. The results indicating that 51 and 63 kDa proteins could be strong candidates for human immunization against VL. In contrast, 34 and 91 kDa proteins demonstrated a reverse profile and may not be a good vaccine candidate.
Conclusions: The preliminary data obtained in this study proposes the potential of some of the antigens in Leishmania diagnosis and for test of cure. Additionally, some antigens demonstrated good immunoprophylactic cytokine production through T cell-mediated immune response, suggesting future vaccine candidates for VL. However, further studies are necessary to explore these antigens in diagnosis and to access the long-term immune response.
Keywords: Biochemistry; Cytokines; Diagnosis; Immunology; Leishmaniasis; Parasitology; Th1/Th2; Vaccination.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



Similar articles
-
Characterization of glycolytic enzymes--rAldolase and rEnolase of Leishmania donovani, identified as Th1 stimulatory proteins, for their immunogenicity and immunoprophylactic efficacies against experimental visceral leishmaniasis.PLoS One. 2014 Jan 24;9(1):e86073. doi: 10.1371/journal.pone.0086073. eCollection 2014. PLoS One. 2014. PMID: 24475071 Free PMC article.
-
Antigenicity of Leishmania-Activated C-Kinase Antigen (LACK) in Human Peripheral Blood Mononuclear Cells, and Protective Effect of Prime-Boost Vaccination With pCI-neo-LACK Plus Attenuated LACK-Expressing Vaccinia Viruses in Hamsters.Front Immunol. 2018 Apr 23;9:843. doi: 10.3389/fimmu.2018.00843. eCollection 2018. Front Immunol. 2018. PMID: 29740446 Free PMC article.
-
A Leishmania amastigote-specific hypothetical protein evaluated as recombinant protein plus Th1 adjuvant or DNA plasmid-based vaccine to protect against visceral leishmaniasis.Cell Immunol. 2020 Oct;356:104194. doi: 10.1016/j.cellimm.2020.104194. Epub 2020 Aug 13. Cell Immunol. 2020. PMID: 32827943
-
DNA vaccine against visceral leishmaniasis: a promising approach for prevention and control.Parasite Immunol. 2016 May;38(5):273-81. doi: 10.1111/pim.12315. Parasite Immunol. 2016. PMID: 27009772 Review.
-
Efficacy of vaccines based on chimeric or multiepitope antigens for protection against visceral leishmaniasis: A systematic review.PLoS Negl Trop Dis. 2024 Dec 31;18(12):e0012757. doi: 10.1371/journal.pntd.0012757. eCollection 2024 Dec. PLoS Negl Trop Dis. 2024. PMID: 39739955 Free PMC article.
Cited by
-
Recombinant Leishmania-activated C kinase as a novel antigenic candidate for immuno-diagnosis of visceral leishmaniasis occurring in India and Brazil.Infect Dis Poverty. 2025 Jul 9;14(1):62. doi: 10.1186/s40249-025-01296-7. Infect Dis Poverty. 2025. PMID: 40635117 Free PMC article.
-
Development of Immunological Assays Based on Leishmania donovani Antigen for Diagnosis of Canine Visceral Leishmaniasis and Their Multicenter Evaluation in Brazil and Italy.Front Cell Infect Microbiol. 2022 Jul 1;12:914477. doi: 10.3389/fcimb.2022.914477. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35846748 Free PMC article.
-
Laboratory Diagnosis of Cutaneous and Visceral Leishmaniasis: Current and Future Methods.Microorganisms. 2020 Oct 22;8(11):1632. doi: 10.3390/microorganisms8111632. Microorganisms. 2020. PMID: 33105784 Free PMC article. Review.
-
Sphingomyelin liposome bearing whole Leishmania lysate antigens induce strong Th2 immune response in BALB/c mice.Iran J Basic Med Sci. 2021 Feb;24(2):222-231. doi: 10.22038/IJBMS.2020.50471.11496. Iran J Basic Med Sci. 2021. PMID: 33953862 Free PMC article.
-
Intranasal vaccine from whole Leishmania donovani antigens provides protection and induces specific immune response against visceral leishmaniasis.PLoS Negl Trop Dis. 2021 Aug 17;15(8):e0009627. doi: 10.1371/journal.pntd.0009627. eCollection 2021 Aug. PLoS Negl Trop Dis. 2021. PMID: 34403413 Free PMC article.
References
-
- Ejazi SA, Bhattacharya P, Bakhteyar MA, Mumtaz AA, Pandey K, Das VN, et al. Noninvasive diagnosis of visceral leishmaniasis: development and evaluation of two urine-based immunoassays for detection of Leishmania donovani infection in India. PLoS Negl Trop Dis. 2016;10:e0005035. doi: 10.1371/journal.pntd.0005035. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

