Neuroleptic malignant syndrome in patients with COVID-19
- PMID: 32473756
- PMCID: PMC7242930
- DOI: 10.1016/j.ajem.2020.05.042
Neuroleptic malignant syndrome in patients with COVID-19
Abstract
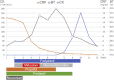
We report the first two cases of Coronavirus Disease 2019 (COVID-19) who were receiving intensive care including favipiravir, and were clinically diagnosed with neuroleptic malignant syndrome (NMS) to focus attention on NMS in COVID-19 management. Case 1: A 46-year-old-man with acute respiratory distress syndrome (ARDS) caused by COVID-19 infection was being administered favipiravir. Fentanyl, propofol, and rocuronium were also given. On day 3, midazolam administration was initiated for deep sedation. On day 5, his high body temperature increased to 41.2 °C, creatine kinase level elevated, and he developed tachycardia, tachypnea, altered consciousness, and diaphoresis. NMS was suspected, and supportive therapy was initiated. High-grade fever persisted for 4 days and subsided on day 9. Case 2: A 44-year-old-man with ARDS caused by COVID-19 infection was being treated with favipiravir. On day 5, risperidone was started for delirium. On day 7, his body temperature suddenly increased to 40.8 °C, his CK level elevated, and he developed tachycardia, tachypnea, altered consciousness, and diaphoresis. NMS diagnosis was confirmed, and both, favipiravir and risperidone were discontinued on day 8. On the same day, his CK levels decreased, and his body temperature normalized on day 9. Patients with COVID-19 infection frequently require deep sedation and develop delirium; therefore, more attention should be paid to the development of NMS in patients who are being administered such causative agents. The mechanism underlying the occurrence of NMS in COVID-19 patients treated with favipiravir remains unknown. Therefore, careful consideration of NMS development is necessary in the management of COVID-19 patients.
Keywords: COVID-19; Fever; Neuroleptic malignant syndrome.
Copyright © 2020 Elsevier Inc. All rights reserved.
Figures
Comment in
-
The authors' response: A diagnostic confusion between serotonin syndrome and neuroleptic malignant syndrome.Am J Emerg Med. 2021 May;43:274. doi: 10.1016/j.ajem.2020.06.048. Epub 2020 Jun 25. Am J Emerg Med. 2021. PMID: 32622686 Free PMC article. No abstract available.
-
Neuroleptic malignant syndrome in COVID-19 patients by Soh et al.Am J Emerg Med. 2021 May;43:259. doi: 10.1016/j.ajem.2020.06.038. Epub 2020 Jun 23. Am J Emerg Med. 2021. PMID: 32650958 Free PMC article. No abstract available.
-
Propofol in COVID 19 - From basic science to clinical impact.Am J Emerg Med. 2021 Jul;45:527. doi: 10.1016/j.ajem.2020.07.011. Epub 2020 Jul 9. Am J Emerg Med. 2021. PMID: 32712236 Free PMC article. No abstract available.
-
Propofol and sedation in patients with coronavirus disease.Am J Emerg Med. 2021 Apr;42:250. doi: 10.1016/j.ajem.2020.06.023. Epub 2020 Jun 18. Am J Emerg Med. 2021. PMID: 32732086 Free PMC article. No abstract available.
-
A diagnostic confusion between Serotonin syndrome and Neuroleptic malignant syndrome.Am J Emerg Med. 2021 May;43:272-273. doi: 10.1016/j.ajem.2020.06.046. Epub 2020 Jun 27. Am J Emerg Med. 2021. PMID: 33008703 Free PMC article. No abstract available.
-
Neuroleptic malignant syndrome following COVID-19 vaccination.Am J Emerg Med. 2021 Nov;49:408-409. doi: 10.1016/j.ajem.2021.02.011. Epub 2021 Feb 20. Am J Emerg Med. 2021. PMID: 33642127 Free PMC article. No abstract available.
References
-
- Richardson S., Hirsch J.S., Narasimhan M., Crawford J.M., McGinn T., Davidson K.W. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020 doi: 10.1001/jama.2020.677. Published online April 22, 2020. - DOI - PMC - PubMed
-
- Diseases hJAfI Case presentations of COVID-19. http://www.kansensho.or.jp/modules/topics/index.php?content_id=31 at.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials