Diagnostic performance of seven rapid IgG/IgM antibody tests and the Euroimmun IgA/IgG ELISA in COVID-19 patients
- PMID: 32473953
- PMCID: PMC7255746
- DOI: 10.1016/j.cmi.2020.05.023
Diagnostic performance of seven rapid IgG/IgM antibody tests and the Euroimmun IgA/IgG ELISA in COVID-19 patients
Abstract
Objectives: To evaluate the diagnostic performance of seven rapid IgG/IgM tests and the Euroimmun IgA/IgG ELISA for antibodies against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in COVID-19 patients.
Methods: Specificity was evaluated in 103 samples collected before January 2020. Sensitivity and time to seropositivity was evaluated in 167 samples from 94 patients with COVID-19 confirmed with RT-PCR on nasopharyngeal swab.
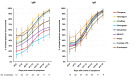
Results: Specificity (confidence interval) of lateral flow assays (LFAs) was ≥91.3% (84.0-95.5) for IgM, ≥90.3% (82.9-94.8) for IgG, and ≥85.4% (77.2-91.1) for the combination IgM OR IgG. Specificity of the ELISA was 96.1% (90.1-98.8) for IgG and only 73.8% (64.5-81.4) for IgA. Sensitivity 14-25 days after the onset of symptoms was between ≥92.1% (78.5-98.0) and 100% (95.7-100) for IgG LFA compared to 89.5% (75.3-96.4) for IgG ELISA. Positivity of IgM OR IgG for LFA resulted in a decrease in specificity compared to IgG alone without a gain in diagnostic performance, except for VivaDiag. The results for IgM varied significantly between the LFAs with an average overall agreement of only 70% compared to 89% for IgG. The average dynamic trend to seropositivity for IgM was not shorter than for IgG. At the time of hospital admission the sensitivity of LFA was <60%.
Conclusions: Sensitivity for the detection of IgG antibodies 14-25 days after the onset of symptoms was ≥92.1% for all seven LFAs compared to 89.5% for the IgG ELISA. The results for IgM varied significantly, and including IgM antibodies in addition to IgG for the interpretation of LFAs did not improve the diagnostic performance.
Keywords: COVID-19; Diagnosis; ELISA; Immunoassay; Lateral flow assay; Point-of-care testing; SARS-CoV-2; Sensitivity and specificity; Seroconversion.
Copyright © 2020 European Society of Clinical Microbiology and Infectious Diseases. Published by Elsevier Ltd. All rights reserved.
Figures
References
-
- Yang Y., Yang M., Shen C., Wang F., Yuan J., Li J. Evaluating the accuracy of different respiratory specimens in the laboratory diagnosis and monitoring the viral shedding of 2019-nCoV infections. medRxiv. 2020 doi: 10.1101/2020.02.11.20021493. - DOI
-
- To K.K.-W., Tsang O.T.-Y., Leung W.-S., Tam A.R., Wu T.-C., Lung D.C. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis. 2020 doi: 10.1016/s1473-3099(20)30196-1. (Epub ahead of print) - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

