Fungal Pathogens: Shape-Shifting Invaders
- PMID: 32474010
- PMCID: PMC7572602
- DOI: 10.1016/j.tim.2020.05.001
Fungal Pathogens: Shape-Shifting Invaders
Abstract
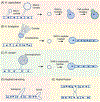
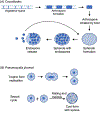
Fungal infections are on the rise due to new medical procedures that have increased the number of immune compromised patients, antibacterial antibiotics that disrupt the microbiome, and increased use of indwelling medical devices that provide sites for biofilm formation. Key to understanding the mechanisms of pathogenesis is to determine how fungal morphology impacts virulence strategies. For example, small budding cells use very different strategies to disseminate compared with long hyphal filaments. Furthermore, cell morphology must be monitored in the host, as many fungal pathogens change their shape to disseminate into new areas, acquire nutrients, and avoid attack by the immune system. This review describes the shape-shifting alterations in morphogenesis of human fungal pathogens and how they influence virulence strategies.
Keywords: capsule; conidia; fungal; hyphae; morphogenesis; pseudohyphae; yeast.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Figures



References
-
- Brown GD et al. (2012) Hidden killers: human fungal infections. Sci Transl Med 4 (165), 165rv13. - PubMed
-
- Gow NA et al. (2002) Fungal morphogenesis and host invasion. Curr Opin Microbiol 5 (4), 366–71. - PubMed
-
- Erwig LP and Gow NA (2016) Interactions of fungal pathogens with phagocytes. Nat Rev Microbiol 14 (3), 163–76. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

