Biomarkers for immunotherapy for treatment of glioblastoma
- PMID: 32474411
- PMCID: PMC7264836
- DOI: 10.1136/jitc-2019-000348
Biomarkers for immunotherapy for treatment of glioblastoma
Abstract
Immunotherapy is a promising new therapeutic field that has demonstrated significant benefits in many solid-tumor malignancies, such as metastatic melanoma and non-small cell lung cancer. However, only a subset of these patients responds to treatment. Glioblastoma (GBM) is the most common malignant primary brain tumor with a poor prognosis of 14.6 months and few treatment advancements over the last 10 years. There are many clinical trials testing immune therapies in GBM, but patient responses in these studies have been highly variable and a definitive benefit has yet to be identified. Biomarkers are used to quantify normal physiology and physiological response to therapies. When extensively characterized and vigorously validated, they have the potential to delineate responders from non-responders for patients treated with immunotherapy in malignancies outside of the central nervous system (CNS) as well as GBM. Due to the challenges of current modalities of radiographic diagnosis and disease monitoring, identification of new predictive and prognostic biomarkers to gauge response to immune therapy for patients with GBM will be critical in the precise treatment of this highly heterogenous disease. This review will explore the current and future strategies for the identification of potential biomarkers in the field of immunotherapy for GBM, as well as highlight major challenges of adapting immune therapy for CNS malignancies.
Keywords: neuroimmunology; neuropathy; neurosurgery; tissue typing; tumors.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
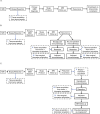
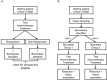
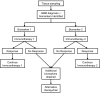
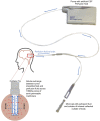
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
