Tocilizumab, but not dexamethasone, prevents CRS without affecting antitumor activity of bispecific antibodies
- PMID: 32474413
- PMCID: PMC7264835
- DOI: 10.1136/jitc-2020-000621
Tocilizumab, but not dexamethasone, prevents CRS without affecting antitumor activity of bispecific antibodies
Abstract
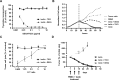
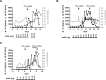
Bispecific antibodies (bsAb) and chimeric antigen receptor (CAR) T cells allow for antibody guided recruitment of T cells against tumors. Both are successfully used for treatment of CD19 expressing leukemias, but may cause cytokine release syndrome (CRS) as a major dose-limiting side effect. For CRS prevention, steroids are recommended prior to bsAb treatment, despite their well-known lymphotoxic activity. The IL-6 receptor antibody tocilizumab is established for treatment of CRS induced by CAR T cells, but was not considered for CRS prevention in bsAb therapy. We here compared the influence of dexamethasone and tocilizumab on bsAb-mediated T cell proliferation and tumor lysis in vitro and in vivo and found that dexamethasone profoundly inhibited T cell proliferation and antitumor activity as induced by two different bsAb, particularly at low effector:target ratios, whereas tocilizumab did not affect efficacy. When we applied tocilizumab early during treatment of three patients with a newly developed PSMAxCD3 bsAb, significant CRS attenuation despite high IL-6 serum levels was observed. Thus, early IL-6 blockade may reduce the undesired sequelae of CRS upon bsAb therapy without affecting therapeutic activity, allowing in turn for safe application of effective doses.
Keywords: antibodies, neoplasm; immunotherapy; lymphocyte activation.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: GJ, HRS, and LZ are listed as inventors on the patent application “Novel PSMA bindingantibody and uses thereof”, EP16151281, applicant German Cancer Research Center, Heidelberg, Germany.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
